Abstract.
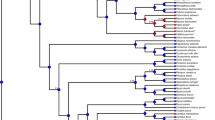
Modification of the cellular prion protein has been correlated with the acquisition of several neurodegenerative diseases, including kuru, scrapie, bovine spongiform encephalopathy (BSE), and Creutzfeldt–Jakob disease (CJD). Sequence conservation and amino acid identity are known to influence the efficacy of interspecific transmission. We analyzed patterns of interspecific genetic variation with a view toward identifying features related to disease transmission. The reconstructed gene trees and amino acid tree were compared with the species tree, and all discordances observed were related to the species barrier of disease transmission. The rates of synonymous substitution, nonsynonymous substitution, and nucleotide content were determined for the protein-coding gene. Substitutions implicated in each of the prion diseases were found to occur in regions of the protein that are least variable across all species—opposite to the pattern of variability expected from interaction with an infectious pathogen. Amino acid residues related to the species barrier form a single cluster associated with the first alpha-helical domain of the protein. Residues related to sporadic and hereditary human prion disease form two separate clusters, associated with the second and third alpha-helical domains. Taken together, these results are consistent with the view that prion diseases arise from accidents in protein folding, rather than infection with an undiscovered virus-like particle. We speculate that the differences in disease phenotype between transmissable and hereditary forms could result from interactions between different parts of the protein during propagation.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 18 April 1997 / Accepted: 17 October 1997
Rights and permissions
About this article
Cite this article
Krakauer, D., de A. Zanotto, P. & Pagel, M. Prion's Progress: Patterns and Rates of Molecular Evolution in Relation to Spongiform Disease. J Mol Evol 47, 133–145 (1998). https://doi.org/10.1007/PL00006370
Issue Date:
DOI: https://doi.org/10.1007/PL00006370