Abstract
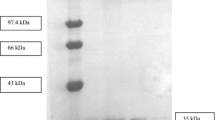
A 1.2 kb lipase gene (AY 78735) from solvent stable and thermostableBacillus sp. strain 42 was overexpressed in a heterologous system that allowed for an extensive characterization of its solvent stability and thermostability. An overexpression was achieved using pET51b vector withEscherichia coli host strain BL21(DE3)pLysS, in which optimum expression was at 22–24 h incubation at 37°C, with lipase activity reached at 80.0 U mL−1 (specific activity 160.0 U mg−1), after induction by 0.5 mM IPTG. This expression was 11.5 fold higher and superseded the pQE-30UA/M15 (pREP4) host-vector system, which only achieved at 17.0 U mL−1 (34.0 U mg−1). The fusion lipase contains N-terminal Strep-tag II affinity tag that in one step of purification, the lipase was purified to homogeneity using Strep-tag II agarose column. The lipase was purified at 1.3 fold and 70% recovery with the elution fraction gave a band of 43 kDa in SDS-PAGE. The purified fusion lipase was most active at 70°C and pH 8.0, and was stable in a broad pH range of 7–10. It showed hydrolysis preference towards olive, sunflower and corn oils. Based on solvent stability studies in 30 min pre-incubation in 25% v/v solvents with a shaking rate at 150 strokes per min, the purified Lip 42 showed a different residual activity profiles depending on solvents and temperatures. Lip 42 was found be stable in polar organic solvents such as DMSO, DMF, acetone, methanol, heptanol and octanol, which could make it as a potential biocatalyst for the use in industrial biodiesel production.
Similar content being viewed by others
References
Abdel-Fattah Y.R., Gaballa A.A. (2008). Identification and over-expression of a thermostable lipase fromGeobacillus thermoleovorans Toshki inEscherichia coli. Microbiol. Res., 163(1):13–20.
Bradford M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 72: 248–254.
Buck M. (1998). Trifuoroethanol and colleagues cosolvents come of age. Recent studies with peptides and proteins. Q. Rev. Biophys., 31: 297–355.
Eltaweel M.A., Rahman R.N.Z., Salleh A.B., Basri M. (2005). An organic solvent stable lipase fromBacillus sp. strain 42. Ann. Microbiol., 55 (3): 187–192.
Fang Y., Lu Z., Lv F., Bie X., Liu S., Ding Z., Xu W. (2006). A newly isolated organic solvent tolerantStaphylococcus saprophyticus M36 produced organic solvent-stable lipase. Curr. Microbiol., 53 (6): 510–515.
Frenken L.G.J., Bos J.W., Visser C., Muller W., Tommassen J., Verrips C.T. (1993). An accessory gene,lipB, required for the production of activePseudomonas glumae lipase. Mol. Microbiol., 9: 579–589.
Fukuda H., Kondo A., Noda H. (2001). Biodiesel fuel production by transesterification of oils. J. Biosci. Bioeng., 92 (5): 405–416.
Gekko K., Timasheff S.N. (1981). Thermodynamic and kinetic examination of protein stabilization by glycerol. Biochemistry, 20 (16): 4677–4686.
Gekko K., Ohmae E., Kameyama K., Takagi T. (1998). Acetonitrile-protein interactions: amino acid solubility and preferential solvation. Biochim. Biophys. Acta, 1387: 195–205.
Gerritse G., Hommes R.W.J., Quax W.J. (1998). Development of a lipase fermentation process that uses a recombinantPseudomonas alcaligenes strain. Appl. Environ. Microbiol., 64 (7): 2644–2651.
Gupta M., Batra R., Tyagi R., Sharma A. (1997). Polarity index: the guiding solvent parameter for enzyme stability in aqueous-organic cosolvent mixtures. Biotechnol. Prog., 13: 284–288.
Haki G.D., Rakshit S.K. (2003). Developments in industrially important thermostable enzymes: a review. Biores. Technol., 89: 17–34.
Hamada D., Goto Y. (2005). Alcohol and salt-induced partially folded intermediates. In: Buchner J., Kiefhaber T., Eds, Protein Folding Handbook, Vol. 2, Wiley-VCH, pp. 884–915.
Kamini N.R., Iefuji H. (2001). Lipase catalyzed methanolysis of vegetable oils in aqueous medium byCryptococcus spp. S-2. Process. Biochem., 37 (4): 405–410.
Karadzic I., Masui A., Zivkovic L.I., Fujiwara N. (2006). Purification and characterization of an alkaline lipase fromPseudomonas aeruginosa isolated from putrid mineral cutting oil as component of metalworking fluid. J. Biosci. Bioeng., 102 (2): 82–89.
Kita Y., Arakawa T., Lin T.-Y., Timasheff S.N. (1994). Contribution of the surface-free energy perturbation to protein-solvent interactions. Biochemistry, 33: 15178–15189.
Klibanov A.M. (2001). Improving enzymes by using them in organic solvents. Nature, 409: 241–246.
Krishna H.S., Haki G.D., Rakshit S.K. (2002). Developments and trends in enzyme catalysis in nonconventional media. Biotechnol. Adv., 20 (3): 239–267.
Kwon D.K., Rhee J.S. (1986). A simple and rapid colorimetric method for determination of free fatty acids for lipase assay. J. Am. Oil Chem. Soc., 63: 89–92, doi: 10.1007/BF02676129.
Laemmli U.K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227: 680–685.
Leow T.C., Rahman R.N.Z.A., Basri M., Salleh A.B. (2004). High level expression of thermostable lipase fromGeobacillus sp. strain T1. Biosci. Biotechnol. Biochem., 68: 96–103, doi: 10.1271/bbb.68.96.
Leow T.C., Rahman R.N.Z.R.A., Basri M., Salleh A.B. (2007). Athermoalkaliphilic lipase ofGeobacillus sp. T1. Extremophiles, 11 (3): 527–735.
Lichty J.J., Malecki J.L., Agnew H.D., Michelson-Horowitz D.J., Tan S. (2005). Comparison of affinity tags for protein purification. Protein Expr. Purif., 41 (1): 98–105.
Long Z.D., Xu J.H., Zhao L.L., Pan J., Yang S., Hua L. (2007). Overexpression ofSerratia marcescens lipase inEscherichia coli for efficient bioresolution of racemic ketoprofen. J. Mol. Catal. B-Enzym., 47 (3): 105–110.
Mosbah H., Sayari A., Bezzine S., Gargouri Y. (2006). Expression, purification, and characterization of His-taggedStaphylococcus xylosus lipase wild-type and its mutant Asp 290 Ala. Protein Expr. Purif., 47 (2): 516–523.
Mozhaev V., Lange R., Kudryashova E.V., Balny C. (1996). Application of high hydrostatic pressure for increasing activity and stability of enzymes. Biotechnol. Bioeng., 52: 320–332.
Muller-Hill B., Crapo L., Gilbert W. (1968). Mutants that make more lac repressor. Proc. Natl. Acad. Sc., USA, 59 (4): 1259–1262.
Nthangeni M.B., Patterton H-G., van Tonder A., Vergeer W.P., Litthauer D. (2001). Over-expression and properties of a purified recombinantBacillus licheniformis lipase: a comparative report onBacillus lipases. Enzyme Microb. Tech., 28 (7): 705–712.
Ogino H., Nakagawa S., Shinya K., Muto T., Fujimura N., Yasuda M., Ishikawa H. (2000). Purification and characterization of organic solvent-stable lipase from organic solvent-tolerantPseudomonas aeruginosa LST-03. J. Biosci. Bioeng., 89 (5): 451–457.
Reetz M., Jaeger K-E. (1998). Overexpression, immobilization and biotechnological application ofPseudomonas lipases. Chem. Phys. Lipids, 93: 3–14.
Schmidt-Dannert C., Sztajer H., Stocklein W., Menge U., Schmid R.D. (1994). Screening, purification and properties of a thermophilic lipase fromBacillus thermocatenulatus. Biochim. Biophys. Acta, 1214: 43–53.
Sekhon A., Dahiya N., Tiwari R.P., Hoondal G.S. (2005). Properties of a thermostable extracellular lipase fromBacillus megaterium AKG-1. J. Basic Microbiol., 45 (2): 147–154.
Sellek G.A., Chaudhuri J.B. (1999). Biocatalysis in organic media using enzymes from extremophiles. Enzyme Microb. Tech., 25: 471–482.
Sharma A.K., Tiwari R.P., Hoondal G.S. (2001). Properties of a thermostable and solvent stable extracellular lipase from aPseudomonas sp. AG-8. J. Basic Microbiol., 41 (6): 363–366.
Skerra A., Schmidt T.G.M. (1999). Applications of a peptide ligand for strepta vidin: the Strep-tag. Biomol. Eng., 16: 79–86.
Sørensen H.P., Mortensen K.K. (2005). Advanced genetic strategies for recombinant protein expression inEscherichia coli. J. Biotech., 115 (2): 113–128.
Studier F.W., Moffatt B.A. (1986). Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol., 189 (1): 113–130.
Studier F.W., Rosenberg A.H., Dunn J.J., Dubendorff J.W. (1990). Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol., 185: 60–89.
Sugihara A., Ueshima M., Shimada Y., Tsunasawa S., Tominaga Y. (1992). Purification and characterization of a novel thermostable lipase fromPseudomonas cepacia. J. Biochem., 112 (5): 598–603.
Timasheff S.N. (1993). The control of protein stability and association by weak interactions with water: how do solvents affect these processes? Annu. Rev. Biophys. Biomol. Struct., 22: 67–97.
Tsuzuki W., Ue A., Kitamura Y. (2001). Effect of dimethylsulfoxide on hydrolysis of lipase. Biosci. Biotechnol. Biochem., 65 (9): 2078–2080.
Vulfson E.Y. (1994). Industrial applications of lipases. In: Woolley P., Petersen S.B., Eds., Lipases: Their Structure, Biochemisîry and Application, Cambridge University Press, London, UK, pp. 271–286.
Yan J., Yang J., Xu L., Yan Y. (2007). Gene cloning, overexpression and characterization of a novel organic solvent tolerant and thermostable lipase fromGalactomyces geothrichum YO5. J. Mol. Catal. B-Enzym., 49: 28–35.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamid, T.H.T.A., Eltaweel, M.A., Rahman, R.N.Z.R.A. et al. Characterization and solvent stable features of Strep-tagged purified recombinant lipase from thermostable and solvent tolerantBacillus sp. strain 42. Ann. Microbiol. 59, 111–118 (2009). https://doi.org/10.1007/BF03175607
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03175607