Abstract
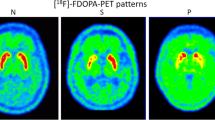
Both the striatal18F-dopa uptake and brain glucose metabolism were studied by PET with 6-l-[18F]fluorodopa (FD) and [18F]fluorodeoxyglucose (FDG) in 9 patients with multiple system atrophy (MSA) and 15 patients with idiopathic Parkinson’s disease (PD). Five of the 9 MSA patients were diagnosed as having olivopontocerebellar atrophy, whereas 2 had striatonigral degeneration and 2 demonstrated Shy-Drager syndrome. The FD uptake ratios to the occipital cortex in the MSA patients at 120 min after the administration of FD were 2.07 ± 0.31 (mean ± SD) and 1.96 ± 0.29 in the caudate and the putamen, respectively, and decreased compared to those in the controls (2.72 ± 0.11, 2.71 ± 0.10). The same ratios in the PD patients were 2.07 ± 0.36 and 1.74 ± 0.24, respectively, which also decreased, but the decreased uptake in the putamen was more prominent. The caudate-putamen index (CPI) (%), which was calculated by a formula based on the difference in the uptakes in the caudate and putamen divided by the caudate uptake, indicated 5.6 ± 4.6 in the MSA patients and 14.8 ± 5.4 in the PD patients. The CPI for all PD patients was more than 7.0, which was the mean + 2SD for the controls, but the CPI for 3 MSA patients was more than 7.0 (accuracy: 88%). The glucose metabolic rates for each region in the PD patients showed no difference from the normal controls. The frontal and the temporal cortical glucose metabolism and the caudate, the putaminal, the cerebellar and the brainstem glucose metabolism in the MSA patients decreased significantly in comparison to those in the controls. But, as the glucose metabolic rates in such regions of each patient overlapped in the two groups, the accuracy of the FDG study for differentiation was lower than that of the FD study. The putaminal glucose metabolic rates, for example, in 3 PD patients were less than 6.8 (mg/min/100 ml), which was the mean — 2SD for the controls, while those in 3 MSA patients were more than 6.8 (accuracy: 75%). In addition, the combination of these two methods slightly improved the accuracy. The glucose metabolism is useful for evaluating the regional metabolic activity of the brain, and the FD study, which is specific to the dopamine system, seems to be more useful for differentiating between MSA and PD.
Similar content being viewed by others
References
Spokes EGS, Bannister R, Oppenheimer DR. Multiple system atrophy with autonomic failure. Clinical, histological, and neurochemical observations on four cases.J Neurol Sci 43: 59–82, 1979.
Kume A, Takahashi A, Hashizume Y, Asai J. A histometrical and comparative study on Purkinje cell loss and olivary nucleus cell loss in multiple system atrophy.J Neurol Sci 101: 178–186, 1991.
Quinn N. Multiple system atrophy—the nature of the beast.J Neurol Neurosurg Psychiatry, special supplement 78–89, 1989.
DeVolder AG, Francart J, Laterre C, Dooms G, Bol A, Michel C, et al. Decreased glucose utilization in the striatum and frontal lobe in probable striatonigral degeneration.Ann Neurol 26: 239–247, 1989.
Eidelberg D, Takikawa S, Moeller JR, Dhawan V, Redington K, Chaly T, et al. Striatal hypometabolism distinguishes striatonigral degeneration from Parkinson’s disease.Ann Neurol 33: 518–527, 1993.
Otsuka M, Ichiya Y, Kuwabara Y, Hosokawa S, Sasaki M, Yoshida T, et al. Glucose metabolism in the cortical and subcortical brain structures in multiple system atrophy and Parkinson’s disease: a positron emission tomographic study.J Neurol Sci 144: 77–83, 1996.
Nahmias C, Garnett ES, Firnau G, Lang A. Striatal dopamine distribution in Parkinsonian patients during life.J Neurol Sci 69: 223–230, 1985.
Martin WRW, Stoessl AJ, Adam MJ, Ammann W, Bergstrom M, Harrop R, et al. Positron emission tomography in Parkinson’s disease: glucose and dopa metabolism.Adv Neurol 45: 95–98, 1986.
Gamett ES, Lang AE, Chirakal R, Firnau G, Nahmias C. A rostrocaudal gradient for aromatic acid decarboxylase in the human striatum.Can J Neurol Sci 14: 444–447, 1987.
Brooks DJ, Ibanez V, Sawle GV, Quinn N, Lees AJ, Mathias CJ, et al. Differing patterns of striatal18F-Dopa uptake in Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy.Ann Neurol 28: 547–555, 1990.
Otsuka M, Ichiya Y, Hosokawa S, Kuwabara Y, Tahara T, Fukumura T, et al. Striatal blood flow, glucose metabolism and18F-Dopa uptake: difference in Parkinson’s disease and atypical Parkinsonism.J Neurol Neurosurg Psychiatry 54: 898–904, 1991.
Adams RD, Victor M. Syndrome of progressive ataxia.In: Principles of Neurology. 4th ed. New York: McGraw-Hill, pp. 946–952, 1989.
Otsuka M, Ichiya Y, Kuwabara Y, Hosokawa S, Akashi Y, Yoshida T, et al. Striatal18F-Dopa uptake and brain glucose metabolism by PET in patients with syndrome of progressive ataxia.J Neurol Sci 124: 198–203, 1994.
Fearnley JM, Lees AJ. Striatonigral degeneration: clinicopathological study.Brain 113: 1823–1842, 1990.
Otsuka M, Ichiya Y, Kuwabara Y, Hosokawa S, Sasaki M, Yoshida T, et al. Differences in the reduced18F-Dopa uptakes of the caudate and the putamen in Parkinson’s disease: correlations with the three main symptoms.J Neurol Sci 136: 169–173, 1996.
Kanno I, Miura S, Yamamoto S, Iida H, Murakami M, Takahashi K, et al. Design and evaluation of the positron emission tomograph: HEADTOME III.J Comput Assist Tomog 9: 931–939, 1985.
Otsuka M, Ichiya Y, Kuwabara Y, Miyake Y, Tahara T, Masuda K, et al. Cerebral blood flow, oxygen and glucose metabolism with PET in progressive supranuclear palsy.Ann Nucl Med 3: 111–118, 1989.
Sokoloff L, Reivich M, Kennedy M, DesRosiers MH, Patlak CS, Pettigrew KD, et al. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: Theory, procedure and normal values in the conscious and anesthetized albino rat.J Neurochem 28: 897–916, 1977.
Phelps ME, Huang SC, Hoffman EJ, Selin C, Sokoloff L, Kuhl DE. Tomographic measurements of local cerebral glucose metabolic rate in humans with [18F]2-fluoro-2-deoxy-d-glucose: Validation of method.Ann Neurol 6: 371–388, 1979.
Brooks RA. Alternative formula forglucose utilization using labeled deoxyglucose.J Nucl Med 23: 538–539, 1982.
Otsuka M, Ichiya Y, Kuwabara Y, Fukumura T, Sasaki M, Masuda K. Evaluation of the ratio method compared with graphical analyses for estimating nigrostriatal function in human18F-dopa PET studies with or without carbidopa.Nucl Med Com 14: 862–867, 1993.
Goto S, Hirano A, Matsumoto S. Subdivisional involvement of nigrostriatal loop in idiopathic Parkinson’s disease and striatonigral degeneration.Ann Neurol 26: 766–770, 1989.
Snow BJ, Tooyama I, MacGee EG, Yamada T, Calne DB, Takahashi H, et al. Human positron emission tomographic [18F]Fluorodopa studies correlate with dopamine cell counts and levels.Ann Neurol 34: 324–330, 1993.
Bemheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations.J Neurol Sci 20: 415–455, 1973.
Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathological and clinical implications.N Engl J Med 318: 876–880, 1988.
Gibb WRG, Lees AJ. Anatomy, pigmentation, ventral and dorsal subpopulations of the substantia nigra, and differential cell death in Parkinson’s disease.J Neurol Neurosurg Psychiatry 54: 388–396, 1991.
Adams RD, van Bogaert L, van der Eecken H. Striato-nigral degeneration.J Neuropathol Exp Neurol 23: 584–608, 1964.
Gibb WRG. Neuropathology of Parkinson’s disease and related syndromes.Neurol Clin 10: 361–376, 1992.
Eidelberg D, Takikawa S, Dhawan V, Chaly T, Robeson W, Dahl R, et al. Striatal18F-DOPA uptake: Absence of an aging effect.J Cereb Blood Flow Metab 13: 881–888, 1993.
Duara R, Margolin RA, Robertson-Tchabo EA, London ED, Schwartz M, Renfrew JW, et al. Cerebral glucose utilization, as measured with positron emission tomography in 21 resting healthy men between 21 and 83 years.Brain 106: 761–775, 1983.
DeLeon MJ, George AE, Ferris SH, Christman DR, Fowler JS, Gentes CI, et al. Positron emission tomography and computed tomography assessments of the aging human brain.J Comput Assist Tomog 8: 88–94, 1984:
Brooks DJ, Salmon EP, Mathias CJ, Quinn N, Leenders KL, Bannister R, et al. The relationship between locomotor disability, autonomic dysfunction, and the integrity of the striatal dopaminergic system in patients with multiple system atrophy, pure autonomic failure, and Parkinson’s disease, studied with PET.Brain 113: 1539–1552, 1990.
Shinotoh H, Inoue O, Hirayama K, Aotsuka A, Asahina M, Suhara T, et al. Dopamine D1 receptors in Parkinson’s disease and striatonigral degeneration: a positron emission tomography study.J Neurol Neurosurg Psychiatry 56: 467–472, 1993.
Burn DJ, Mathias CJ, Quinn N, Marsden CD, Brooks DJ. Striatal opiate receptor binding in Parkinson’s disease and multiple system atrophy:11C-diprenorphine study.Neurology 43 (suppl): S454, 1992.
Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality.Neurology 12: 427–442, 1967.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Otsuka, M., Kuwabara, Y., Ichiya, Y. et al. Differentiating between multiple system atrophy and Parkinson’s disease by positron emission tomography with18F-dopa and18F-FDG. Ann Nucl Med 11, 251–257 (1997). https://doi.org/10.1007/BF03164771
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03164771