Abstract
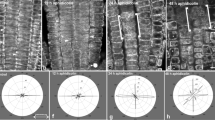
Aluminum (Al) induces agricultural problems limiting crop productivity in acid soils. Since Al causes morphological changes in roots, and because microtubules (MTs) play important roles in determination of tissue morphology, we investigated whether Al affects the arrangement of MTs in maize root meristem using immunolocalization techniques. When seedling roots were treated with 50 μM Al, the orientations of MTs were dramatically altered in a population of cells located in the protoderm and the two outer layers of cortex: interphase cortical MT arrays lost their normal transverse organization and became random or longitudinal; the preprophase band of MTs, mitotic spindle, and phragmoplast developed at planes 90° rotated compared to their counterparts in controls. These changes in MT orientation resulted in the change of the division plane from transverse to longitudinal, producing daughter cells positioned side by side instead of above and below. The rotation of the otherwise normal MT arrays and the division plane in Al-treated roots indicates that Al interferes with the normal polarity sensing mechanism, which may contribute to the reduced axial growth of the Al-treated roots.
Similar content being viewed by others
Literature Cited
Adams, A.E.M., D. Botstein and D.G. Drubin. 1991. Requirement of yeast fimbrin for actin organization and morphogenesisin vivo.Nature354: 404–408.
Baluska, F., J.S. Parker and P.W. Bartow. 1992. Specific patterns of cortical and endoplasmic microtubules associated with cell growth and tissue differentiation in roots of maize (Zea mays L).J. Cell Sci.103: 191–200.
Bartow, P.W. and J.S. Adam. 1989. Experimental control of cellular patterns in the cortex of tomato roots. In Structural and Functional Aspects of Transport in Roots, BC Loughmanet al. eds (Amsterdam: Kluwer Academic Publishers), pp. 21–24.
Baskin, T.I. and R.E. Williamson. 1992. Ethylene, microtubules and root morphology in wild-type and mutantArabidopsis seedlings. Current Top.Plant Biochem. Physiol.11: 118–130.
Bouget, F.-Y., S. Gerttula, S.L. Shawand R.S. Quatrano. 1996. Localization of actin mRNA during the establishment of cell polarity and early cell divisions in Fucus embryos.Plant Cell8: 189–201.
Cho, S.O. and S.M. Wick. 1990. Distribution and function of actin in the developing stomatal complex of winter rye (Secale Cereale cv. Puma)Protoplasma157: 154–164.
Clarkson, D.T. 1965. The effect of aluminum and some other trivalent metal cations on cell division in the root apices ofAllium cepa.Annals of Botany NS29: 309–315.
Cruz-Ortega, R., J.C. Cushman and J.D. Ownby. 1997. cDNA clones encoding 1,3-glucanase and a fimbrin-like cytoskeletal protein are induced by Al toxicity in wheat roots.Plant Physiol.114: 1453–1460.
Cyr, RJ. and BA. Palevitz. 1995. Organization of cortical microtubules in plant cells.Curr. Opin. Cell Biol.7: 65–72.
Doll, R. 1993. Alzheimer’s disease and environmental aluminum.Age aging22: 138–153.
Ecker, J.R. 1995. The ethylene signal transduction pathway in plants.Science268: 667–675.
Eeckhaoudt, S., D. Vandeputte, H. Van Praag, R. Van Grieken and W. Jacob 1992. Laser microprobe mass analysis (LAMMA) of aluminum and lead in fine roots and their ectomycorrhizal mantles of Norway spruce (Picea abies (L.) Karst.)Tree Physiol.10: 209–215.
Foy, CD., R.L. Chaney and M.V. White 1978. The physiology of metal toxicity in plants.Annu. Rev. Plant Physiol.29: 511–566.
Foy, CD. 1988. Plant adaptation to acid, aluminum toxic soils.Commun. Soil Sci. Plant Anal.19: 959–987.
Grabski, S. and M. Schindler. 1995. Aluminum induces rigor within the actin network of soybean cells.Plant Physiol.108: 897–901.
Hardham, A.R. and M.E. McCully. 1982. Repro-gramming of cells following wounding in pea roots. I: Cell division and differentiation of new vascular elements.Protoplasma112: 143–151.
Horst, W.J., A. Wagner and H. Marscher. 1983. Effect of aluminum on root growth, cell-division rate and mineral element contents in roots ofVigna ungui-culata genotypes.Zeitschrift fur Pflanzenphysiologie109: 95–103.
Huang, J.W. and L.V. Kochian. 1992. Aluminum effects on the kinetics of calcium uptake into cells of the wheat root apex.Planta188: 414–421.
Huang, J.W., D.M. Pellet, LA. Papernik and L.V. Kochian. 1996. Aluminum interactions with voltage-dependent calcium transport in plasma membrane vesicles isolated from roots of aluminum-sensitive and -resistant wheat cultivars.Plant Physiol.110: 561–569.
Hush, J.M., C.R. Hawes and R.L. Overall. 1990. Interphase microtubule re-orientation predicts a new cell polarity in wounded pea roots.J. Cell Sci.96: 47–61.
Hush, J.M. and R.L. Overall. 1996. Cortical microtubule reorientation in higher plants: dynamics and regulation.J. Microscopy181: 129–139.
Johnson, RE. and WA Jackson. 1964. Calcium uptake and transport by wheat seedlings as affected by aluminum.Proc. Soil. Sci. Soc. Am.28: 381–386.
Jones, D.L. and L.V. Kochian. 1995. Aluminum inhibition of the 1,4,5-trisphosphate signal transduction pathway in wheat roots: A role in aluminum toxicity?Plant Cell7: 1913–1922.
Lang Selker, J.M. and P.B. Green. 1984. Organogenesis inGraptopetalum paraguayense E. Walther: shifts in orientation of cortical microtubule arrays are associated with periclinal divisions.Planta160: 289–297.
Lintilhac, P.M. and T.B. Vesecky. 1984. Stress-induced alignment of division plane in plant tissues grownin vitro.Nature307: 363–364.
Macdonald, T.L., W.G. Humphreys and R.B. Martin. 1987. Promotion of tubulin assembly by aluminum ionin vitro.Science236: 183–186.
Oud, J.L. and N. Nanninga. 1992. Cell shape, chromosome orientation and the position of the plane of division inVicia faba root cortex cells.J. Cell Sci.103: 847–855.
Palevitz, BA. and P.K. Hepler. 1974, The control of the plane of division during stomatal differentiation inAllium. I. Spindle reorientation.Cromosoma46: 297–326.
Quatrano, R.S. and S.L. Shaw. 1997. Role of the cell wall in the determination of cell polarity and the plane of cell division inFucus embryos.Trends Plant Sci.2: 15–21.
Rhyu, M.S. and JA. Knoblich. 1995. Spindle orientation and asymmetric cell fate.Cell82: 523–526.
Rincon, M. and R.A. Gonzales. 1992. Aluminum partitioning in intact roots of aluminum-tolerant and aluminum-sensitive wheat (Triticum aestivum L.) cultivars.Plant Physiol.99: 1021–1028.
Ryan, P.R., J.M. DiTomaso and L.V. Kochian. 1993. Aluminum toxicity in roots. An investigation of spatial sensitivity and the role of the root cap.J. Exp. Bot.44: 437–446
Sasaki, M., Y. Yamamoto and H. Matsumoto. 1996. Lignin deposition induced by aluminum in wheat (Triticum aestivum) roots.Physiol. Plant96: 193–198.
Sasaki, M., Y. Yamamoto and H. Matsumoto. 1997. Aluminum inhibits growth and stability of cortical microtubules in wheat (Triticum aestivum) roots.Soil Sci. Plant Nutr.43: 469–472.
Smith, L.G. 1996. What is the role of cell division in leaf development?Seminars in Cell & Developmental Biology7: 839–848.
Taylor, G.J. 1991. Current views of the aluminum stress response.Curr. Top. Plant Biochem. Physiol.10: 57–93.
Tepper, H.B., C.S. Yang and M. Schaedle. 1989. Effect of aluminum on growth of root tips of honeylocust and loblolly pine.Environ. Exp. Bot.29: 165–173.
Van, H.L., S. Kuraishi and N. Sakurai. 1994. Aluminum-induced rapid root inhibition and changes in cell-wall components of squash seedlings.Plant Physiol.106: 971–976.
Way, J.C., L. Wang, J.Q. Run and M.S. Hung. 1994. Cell polarity and the mechanism of asymmetric cell division.BioEssays16: 935–931.
Went, F.W. and K.V. Thimann. 1937. Phytohormones. New York: Macmillan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, S., Lee, Y. Aluminum induces changes in the orientation of microtubules and the division plane in root meristem of Zea mays. J. Plant Biol. 41, 269–276 (1998). https://doi.org/10.1007/BF03030327
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03030327