Abstract
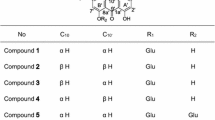
The structural analogues of cumambrin B (1, 2, 3, 4) were isolated from the flower ofChrysanthemum boreale Makino. The structures of compounds were determined by two-dimensional1H-1H COSY and13C-1H COSY spectra with the aid of homonuclear and heteronuclear double resonance experiment. The stereochemistry of compounds has been verified from single crystal X-ray diffraction of cumambrin A (2). The antimicrobial activities of these guaianolides have been studied.
Similar content being viewed by others
References Cited
Bohlmann, F., Arnot, C., and Bornowski, H., Polyacetylene compounds. Part 28. Several polyacetylenes from the tribe anthemidease L..Chem. Ber., 93, 1937–1944 (1960).
Bohlmann, F., Zdero, C., King, R. M., and Robinson, H., Sesquiterpene lactones fromEremanthus species.Phytochemistry, 19, 2663–2668 (1980).
Chabbert, Y. A., L+antibiogramme (Tourelle Saint Mande, ed.) Paris (1963).
Davies-Coleman, M. T., English, R. B. and Rivett, D. E. A., Bitter guaianolides fromEriocephalus punctulatus.Phytochemistry, 31, 2165–2167 (1992).
He, Y. Q., Li, R. Z. and Shen, L., Separation and identification of flavonoids from the flower ofChrysantheum indicum.Peiching Hsueh Yuan Hsueh Pao, 14, 259–261 (1982).
Hoffmann, H. M. R. and Rabe, J., Synthesis and biological activity of α-methylene-γ-butyrolactones.Angew. Chem. Int. Ed. Engl., 24, 94–110 (1985).
Holt, R.,J. Clin. Path. 28, 767–774 (1975).
Hung, K. C.,The Pharmacology of Chinese herbs, CRC press, Boca Raton, 1933, p. 74.
Haruna, M., Kato, M., Ito, K., Nikai, T., Sugihara, H. and Murata, H., Angeloylcumambrin-B, an antimicrobial sesquiterpene lactone fromChrysanthemum ornatum var.spontaneum. Phytochemistry, 2583–2584 (1981).
Michael, T., Robin, B., E. and Dougulas E. A., R.,Bitter guaianolides from Eriocephalus punctulatus.Pytochemistry, 31, 2165–2167 (1992).
Perry, L. M.,Medicinal Plants of East and Southeast Aisia: Attributed Properties and Use, The MIT press, Cambridge, 1980, p. 90.
Yang, M. S., Park, K. H., Jang, D. S., Choi, S. U., Nam, S. H. and Shiro, M., Cumambrin A inChrysanthemum boreal Makino preparation, X-ray crystal structure and C- and H-NMR study of cumambrin A.Kor. J. Pharmacogn, 27, 207–211 (1996).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jang, D.S., Yang, M.S., Ha, T.J. et al. Structural analogues of cumambrin B from the flower ofChrysanthemum boreale . Arch. Pharm. Res. 21, 591–594 (1998). https://doi.org/10.1007/BF02975380
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02975380