Abstract
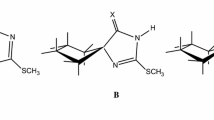
The tautomerism in 3-phenyl-4-arylazo-5-isoxazolones1 was examined by1H-NMR spectra of15N-labeled compound and by HMO method. Both spectra data (1H-NMR and IR) and bonding energies are in support of the assignment of the hydrazone structure to such compounds. It is further shown that intermolecular and intramolecular hydrogen bondings favor the hydrazone tautomer.
Similar content being viewed by others
Literature Cited
Shawali, A. S., Ali, M. I., Naoum, M. M. and Elansari, A. L.: The structure of the diazonium coupling products of sulfones,Tetrahedron,28, 3805 (1972).
Shawali, A. S., Dewidar, A. M. and Naoum, M. M.: Spectroscopic study of the diazonium coupling products of arylacetanilides.Indian J. Chem.,10, 464 (1972).
Shawali, A. S., Mansour, A. K., Abbas, I. M. and Taha, A. A.: Tautomerism in analogs of potential antidiabetics. 5-Arylazo-4-hydroxy-1-arylpyrazole-3-carboxanilides,Indian J. Chem.,12, 298 (1974).
Khattab, S. A., Shawali, A. S. and Farag, A. M.: Structure of the diazonium coupling products of γ-phenyl-β, γ-butenolide.J. Chem. Eng. Data.,24, 104 (1977).
Parkanyi, C., and Shawali, A. S.: An HMO study of azo-hydrazone tautomerism in diazonium coupling products of 3-methyl-5-isoxazolones.J. Heterocycl. Chem., 17, 897 (1980).
Shawali, A. S., Hassaneen, H. M. and Hanna, M. A.: Substituent effects on acidities and tautomeric structure of 1-aryl-3-ethoxy-carbony-4-pyrazolones and their 5-arylazo derivaties,Heterocycles.15, 697 (1981).
Shawali, A. S., Abbas, I. M., Abdelfattah, N. F. and Parkanyi, C.: A theoretical study of tautomerism in dehydroascorbic acid osazone and related compounds.J. Carbohydrate Research,110, 1 (1982).
Shawali, A. S., Abdelhamid, A. O. and Ahmad, N. F.: A study of the structure of 4-arylazo derivatives of 2-phenyl-5-oxazolones,Heterocycles,19, 2331 (1982).
Shawali, A. S., Harb, N. M. S. and Badahdah, K. O.: A study of tautomerism in diazonium coupling products of 4-hydroxycoumarin,J. Heterocycl. Chem.,22, 1397 (1985).
Imperial Chem. Industries Limited, Fungicidal isoxazolinones, Belgian, p. 617,389 (1962):Chem. Abstr.,57, 6413c (1963).
Allan, F. J. and Allan, G. G.: 1-Hydroxyimidazoles,Chem. Ind. (London), 1837 (1964).
Summers, L. A., Freeman, P. F. H. and Shields, D. J.: Structure of 3-alkyl-4-arylazoisoxazol-5-ones and related compounds,J. Chem. Soc., 3312 (1965).
Summers, L. A.: Comment on the structure of 3-alkyl-4-arylazo-isoxazol-5-one,Experientia,22, 499 (1966).
Cum, G., Lo Vecchio, G. and Aversa, M. C.: Structure of 4-benzenazo-5-isoxazolones,Gazz. Chim. Ital.,95, 5583 (1965).
Cum, G., Lo Vecchio, G., Aversa, M. C. and Crisafulli, M.: Sulla struttura dei 4-arilazo-5-isossazoloni. Note II. Spettri infrarossi di risonanza nucleare magnetica,Gazz. Chim. Ital.,97, 346 (1967).
Elguero, J., Marzin, C., Katritzky, A. R. and Linda, P.: The tautomerism of heterocycles,advances in Heterocyclic Chemistry, Supplement 1, Katritzky, A. R. and Boulton, A. J., Eds., Academic Press, N.Y., 1976, pp. 306–307.
Lestina, G. H. and Regan, T. H.: The determination of the azohydrazone tautomerism of some 2-pyrazolin-5-one dyes by means of nuclear magnetic resonance spectroscopy and15N-labeled compounds,J. Org. Chem.,34, 1685 (1969).
Snavely, F. A. and Yoder, C. H.: A study of tautomerism in arylazopyrazolones and related heterocycles with nuclear magnetic resonance spectroscopy.J. Org. Chem.,33, 513 (1968).
Arriau, J., Campillo, J. P., Elguero, J. and Pereilo, J. M.: Etude par des methoden semi-empiriques de la chimie theorique dan laserie de pyrazolones-VII sur le problme de la tautomerie des phenylazopyrazolones.Tetrahedron. 30, 1345 (1974).
Yasuda, H. and Midprikawa, H.: A study of tautomerism in arylazopyrazolones and related heterocycles with nuclear magnetic resonance spectroscopy.J. Org. Chem.,31, 1722 (1966).
Streitwieser, A.:Molecular Orbital Theory for Organic Chemists, Wiley, New York, 1961.
(a) Insole, J. M. and Lewis, E. S.: A tracer demonstration of a reversible step in diazonium salt decomposition.J. Am. Chem. Soc.,85, 122 (1963); (b) Lewis, E. S. and Insole, J. M.: The reactions of diazonium salts with nucleophiles. X. A tracer demonstration of the reversible step in diazonium ion hydrolysis.J. Am. Chem. Soc.,86, 32, (1964).
Bose, A. K. and Kugajevsky, I.: NMR spectral studies-IV. Some15N-H coupling constants,Tetrahedron.23, 1489 (1967).
Bose, A. K. and Kugajevsky, I.: Nuclear magnetic resonance spectroscopy III. Structure of phenyldiazonium ion from15N-H coupling study,J. Am. Chem. Soc.,88, 2325 (1966).
Baird, N. C. and Whitehead, M. A.: Molecular orbital calculations for conjugated molecules containing boron and nitrogen.Can J. Chem.,45, 2059 (1967).
Kuder, J. E.: HMO consideration of factors affecting tautomerism in hydroxyazo compounds,Tetrahedron,28, 1973 (1972).
Geiger, W. E. Jr, and Gulick, W. M., Jr.: Oxygen-17 semiquinones. The effect of fluorine substitution,J. Am. Chem. Soc.,91, 4657 (1969).
Pullman, B. and Pullman, A.:Quantum Biochemistry, Interscienc, New York, pp. 62–117, 1963.
(a) Claisen, L. and Zedel, W.: Uber phenylisoxazolone,Chem. Ber.,24, 140 (1891); (b) Sawdey, G. W., Rearrangement of 4-arylazo-2-phenyloxazolin-5-ones. A new synthesis of1H-1,2,4-triazoles.J. Am. Chem. Soc.,79, 1955 (1957).
Browne, E. J. and Polya, J. B.: Triazoles, Part VI. 1,5-Diaryl-1,2,4-triazole-3-aldehydes,J. Chem. Soc., 575 (1962).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shawali, A.S., Alkaabi, S.S. & Abdallah, M.A. A study of azo-hydrazone tautomerism in 3-phenyl-4-arylazo-5-isozaolones by1H-NMR spectra of15N-labeled compounds and HMO method. Arch. Pharm. Res. 14, 237–241 (1991). https://doi.org/10.1007/BF02876862
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02876862