Abstract
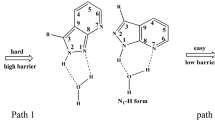
AM1 method is employed to calculate the barriers against intramolecular proton transfer (IPT) of perylenequinone (PQ). The results obtained are as follows: (i) Barriers against the IPT reaction of ground state, singlet excited state and triplet excited state of PQ are 89.75, 55.40 and 83.97 kJ/mol, respectively. (ii) Barriers a-gainst the IPT process of anion of PQ in ground state and singlet excited state are 80.12 and 79. 91 kJ/mol, respectively, (iii) Barriers against the IPT of cation and anion radical of PQ (PQ,+ and PC,-) are 65. 94 and 59.29 kJ/ mol. (iv) The barrier against double proton transfer of PQ is 172.13 kJ/mol. (v) Two barriers against IPT of a type of perylenequinonoid photosensitizer (PQP), hypocrellin A (HA), are 89. 24 and 88. 07 kJ/mol. From these data conclusions can be drawn as follows: (i) IPT processes in ground state and excited state of PQ exist, but the transfer rate of excited state is much higher than that of ground state. (ii) It is almost impossible for PQ to transfer two protons simultaneously. (iii) IPT processes in anion, anion radical and cation radical of PQ still exist. (iv) The seven-membered side ring of HA has no marked influence on its barrier against IPT.
Similar content being viewed by others
References
Diwu, Z., Novel therapeutic and diagnostic applications of hypocrellins and hypericins,Photochem. Photobiol., 1995, 61(6): 529.
Hu, Y. Z., An, J. Y., Jiang, L. J. et al., Spectroscopic study on the photoreduction of hypocrellin A: generation of semiquinone radical anion and hydroquinone,J. Photochem. Photobiol., A:Chem., 1995, 89: 45.
Diwu, Z., Lown, J. W., Photosensitization with anticancer agents (15) —Perylenequinonoid pigments as potential photodynamic therapeutic agents: formation of semiquinone radicals and reactive oxygen species on illumination,J. Photochem. Photobiol. B:Biol., 1993, 18: 131.
Gai, F., Fehr, M. J., Petrich, J. W., Observation of excited-state tautomerization in the antivirul agent hypericin and identification of its fluorescent species,J. Phys. Chem., 1994, 98: 5784.
Gajewski, J. J., Gillbert, K. E., Mckelvey, J, MMX: an enhanced version of MM2,Adv. Mol. Model, 1990, 2: 65.
Dewar, M. J. S., Zoebisch, E. G., Healy, E. F. et al., AM1: a new general purpose quantum mechanical molecular model,J. Am. Chem. Soc., 1985, 107: 3902.
Rossetti, R., Haddon, R. C., Brus, L. E., Intramolecular proton tunnelling in the ground and lowest excited singlet states of 9-hydroxyphenalenone,J. Am. Chem. Soc., 1980, 102: 6913.
Kunze, K. L., de la Vega, J. R., Intramolecular proton exchange in 9-hydroxyphenalen-1-one and methyl-9-hydroxyphenalen-1-one,J. Am. Chem. Soc., 1984, 106(22): 6528.
Diwu, Z., Jiang, L., Zhang, M. et al., The stereochemistry of hypocrellin A,Chin. Sci. Bull. (in Chinese), 1989, 34(14): 1073.
Zhang, H., Zhang, Z., Molecular mechanics study on conformation of perylenequinonoid photosensitizer,Science in China (in Chinese), Ser. B, 1997, 27(4): 358.
Zhang, H., Zhang, Z., Determination of pK a values of perylenequinonoid photosensitizer in excited state,Chin. Sci. Bull. (in Chinese), 1997, 42: 1428.
Yamazaki, T., Ohta, N., Yamazaki, I. L. et al., Excited-state properties of hypericin-electronic spectra and fluorescence dacay kinetics,J. Phys. Chem., 1993, 97: 7870.
Freeman, D., Frolow, F., Kapinus, E. et al., Acidic properties of hypericin and its octahydroxy analog in the ground and excited states,J. Chem. Soc. Chem. Commun., 1994: 891.
Diwu, Z., Lown, J. W., Photosensitization with anticancer agents (14)—Perylenequinonoid pigments as new potential photodynamic therapeutic agents: formation of tautomeric semiquinone radicals,J. Photochem. Photobiol. A:Chem, 1992, 69: 191.
Author information
Authors and Affiliations
Additional information
Project supported by the National Natural Science Foundation of China (Grant No. 39470184).
Rights and permissions
About this article
Cite this article
Zhang, H., Zhang, Z. Theoretical study of intramolecular proton transfer of perylenequinone. Sc. China Ser. B-Chem. 41, 85–90 (1998). https://doi.org/10.1007/BF02875562
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02875562