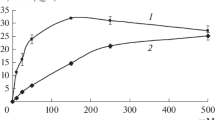
Abstract
Intact cellsEscherichia coli CCM 2843, exhibiting substantial benzylpenicillin amidase activity, were bound mutually with supporting waste microbial cells, native or treated, to obtain an inexpensive biocatalyst for the production of 6-aminopenicillanic acid (6-APA). The bond was effected by glutaraldehyde (GA) and Sedipur CL-930 (PEI), without any carrier. The optimal concentration of GA was 2%, that of PEI 1%. The optimal biocatalyst was obtained by immobilization of productive cells with their fragments at a mass ratio of 4∶1. The cell aggregates were used for hydrolysis of potassium benzyl-penicillin at a concentration of 5 % to 6-APA. After 25 repeated batch conversions the degree of conversion did not decrease; its average value was 96.4%.
Similar content being viewed by others
References
Abbot B.J.: Preparation of pharmaceutical compounds by immobilized enzymes and cells, p. 205 inAnn. Report Ferment. Processes, Vol. 1 (D. Perlman, Ed.). Academic Press, New York 1977.
Balasingham K., Warburton D., Dunnill P., Lilly M.D.: The isolation and kinetics of penicillin amidase fromEscherichia coli.Biochim. Biophys. Acta 276, 250–256 (1972).
Čulík K., Vojtíšek V., Zeman R., Bárta M., Pelzbauer J.: Way of semicontinual production of 6-aminopenicillanic acid.Czech. Pat. 201 621 (1980).
Dinelli D.: Fibre-entrapped enzymes.Process Biochem. 7, 9 (1972).
Durand G., Navarro J.M.: Immobilized microbial cells.Process Biochem. 13, 14–23 (1978).
Chibata I.: Applications of immobilized microbial cells for production ofl-amino acids.Hind. Antibiot. Bull. 20, 58–67 (1978).
Jack T.R., Zajic J.E.: The immobilization of whole cells.Adv. Biochem. Eng. 5, 125–145 (1977)
Karube I., Suzuki S., Vandamme E.J.: Antibiotic production with immobilized living cells, p. 761 inDrugs and Pharmaceutical Sciences, Vol. 22 (E.J. Vandamme, Ed.), Marcel Dekker, New York 1984.
Klein J., Wagner F., Washausen P., Eng H., Martin C.K.A.: Formation of 6-aminopenicillanic acid from penicillin G by immobilized cellsEscherichia coli ATCC 11 105, p. 190 inPreprintes 1st Eur. Congr. Biotechnol., Part 2. Interlaken (Switzerland) 1978.
Klein J., Wagner F., Eng H., Vorlop K.D.: Verfahren zur Herstellung von mechanisch und chemisch stabilen porösen Biokatalysatoren mit hoher enzymatischer Aktivität und perlformiger Biokatalysator.Ger. Pat. 2 835 874 (1980).
Nelson R.P.: Immobilisierung mikrobiologischers Zellen.Ger. Pat. 2 513 929 (1976).
Park J.M., Choi C.Y., Seong B.L., Han M.H.: The production of 6-aminopenicillanic acid by a multistage tubular reactor packed with penicilin amidase.Biotechnol. Bioeng. 24, 1623–1637, (1982a).
Park J.M., Choi C.A., Seong B.L., Han M.H.: Effect of mass transfer in a recirculation batch reactor system for immobilized penicillin amidase.Biotechnol. Bioeng. 24, 2215–2226 (1982b).
Sato T., Tosa T., Chibata I.: Continuous production of 6-aminopenicillanic acid from penicillin by immobilized microbial cells.Eur. J. Appl. Microbiol. 2, 153–160 (1976).
Savidge T.A.: Enzymatic conversions used in the production of penicillins and cephalosporins, p. 171 inDrugs and the Pharmaceutical Sciences, Vol. 22 (E.J. Vandamme, Ed.), Marcel Dekker, New York 1984.
Svátek E.: Way of assay of 6-aminopenicillanic acid in a fermentation medium.Czech. Pat. 116 959 (1965).
Vojtíšek V., Slezák J.: Penicillinamidohydrolase inEscherichia coli. II. Synthesis of the enzyme, kinetics and specificity of its induction and the effect of O2.Folia Microbiol. 20, 289–297 (1975a).
Vojtíšek V., Slezák J.: Penicillinamidohydrolase inEscherichia coli. III. Catabolite repression, diauxie, effect of cAMP and nature of the enzyme inductions.Folia Microbiol. 20, 298–306 (1975b).
Vojtíšek V., Slezák J., Čulík K.: Way of attachment of mutants of microorganisms producing penicillin acylase, especiallyE. coli. Czech. Pat. 162 274 (1976).
Vojtíšek V., Sikyta B., Čulík K., Bučko M.: Species of the microorganismEscherichia coli CCM 2843.Czech. Pat. 188 631 (1978).
Vojtíšek V., Zeman R., Bárta M., Čulík K., Chaloupka J., Kálal J., Drobník J., Švec F.: Microbial cell-aggregates to the conversion of penicillin to 6-aminopenicillanic acid and the way of their preparation.Czech. Pat. 197 101 (1979a).
Vojtíšek V., Zeman R., Bárta M., Čulík K., Drobník J., Švec F.: Bound microbial cells as industrial biocatalysts.Biol.listy 44192–211 (1979b).
Vojtíšek V., Zeman R., Bárta M., Čulík K.: Way of production of immobilized cells with penicillin acylase activity.Czech. Pat. 203 607 (1980).
Vojtíšek V., Jirků V.: Immobilized cells.Folia Microbiol. 28, 309–340 (1983).
Žůrková E., Drobník J., Kálal J., Švec F., Tyráčková V., Vojtíšek V., Zeman R.: Immobilization ofEscherichia coli with penicillin-amidohydrolase activity on solid polymeric carriers.Biotechnol. Bioeng. 25, 2231–2242 (1983).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zeman, R., Vojtíšek, V. Cell aggregates ofEscherichia coli with benzylpenicillin amidase activity. Folia Microbiol 36, 375–382 (1991). https://doi.org/10.1007/BF02814512
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02814512