Abstract
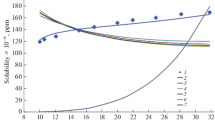
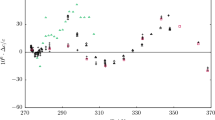
Analysis is made of the experimental data available from the literature on the solubility of ice and water in various compressed gases in a wide range of temperature and pressure. A new formula is derived that relates the equilibrium concentration xeq of steam in moist gas at preassigned temperature and pressure to the constants of the equations of state for condensed water and to the virial coefficients. This formula enables one to directly calculate the solubility of steam in compressed gas without using the previously suggested solution of the set of equations. The derived formula makes it possible to describe the entire array of experimental data in the range of parameters from 200 to 400 K and from 0.1 to 10 MPa with an error equal to or less than the experimental error
Similar content being viewed by others
References
Rabinovich, V.A. and Beketov, V.G.,Moist Gases: Thermodynamic Properties, New York: Begell House, 1995.
Hyland, R.W. and Wexler, A.,J. Res. Natl. Bur. Stand. Sect. A, 1973, vol. 77, no. 1, p. 133.
Hyland, R.W.,J. Res. Natl. Bur. Stand. Sect. A, 1975, vol. 79, no. 4, p. 551.
Iomtev, M.B., Piskunov, V.G., Kadzhaev, V.L.,et al., Sistema gaz-lyod. Rastvorimost’ l’da v azote i vozdukhe v diapazone temperatur ot -50°C do -2°C i davlenii ot 0.2 MPa do 61 MPa. Tablitsy RSD-GSSSD R 88-84 (The Solubility of Ice in Nitrogen and Air in the Range of Temperatures from -50°C to -2°C and Pressures from 0.2 MPa to 61 MPa: Tables of Recommended Reference Data-State Service of Standard and Reference Data R 88-84), Moscow: VNITs MV Gosstandarta SSSR (All-Union Research Center for Standardization and Certification of Raw Materials and Substances, USSR State Committee for Standards), 1984. Available from VNIIKI (All-Union Research Inst. of Space Studies), 1984, Moscow, no. 220.
Rigby, M. and Prausnitz, J.M.,J. Phys. Chem., 1968, vol. 72, no. 1, p. 330.
Namiot, A.Yu.,Rastvorimost’ gazov v vode (Solubility of Gases in Water), Moscow: Nedra, 1991.
Kosyakov, N.E., Ivchenko, B.I., and Krishtopa, P.P.,Zh. Prikl. Khim., 1977, no. 11, p. 2568.
Rabinovich, V.A., Beketov, V.G., Iomtev, M.B.,et al., Sistema gaz-lyod. Rastvorimost’ l’da v vodorode v diapazone temperatur -30.0°C i davlenii 0.2.30 MPa. Tablitsy RSD-GSSSD R 348-89 (The Solubility of Ice in Hydrogen in the Range of Temperatures from -30°C to 0°C and Pressures from 0.2 MPa to 30 MPa: Tables of Recommended Reference Data-State Service of Standard and Reference Data R 348-89), Moscow: VNITs MV Gosstandarta SSSR (All-Union Research Center for Standardization and Certification of Raw Materials and Substances, USSR State Committee for Standards), 1989. Available from VNIIKI (All-Union Research Inst. of Space Studies), 1990, Moscow, no. 615.
Iomtev, M.B., Piskunov, V.G., Kadzhaev, V.L.,et al., Sistema gaz-lyod. Rastvorimost’ l’da v gelii v diapazonakh temperatur ot -50°C do -2°C i davlenii ot 1.01 MPa do 35.5 MPa. Tablitsy RSD-GSSSD R 243-87 (The Solubility of Ice in Helium in the Range of Temperatures from -50°C to -2°C and Pressures from 1.01 MPa to 35.5 MPa: Tables of Recommended Reference Data-State Service of Standard and Reference Data R 243-87), Moscow: VNITs MV Gosstandarta SSSR (All-Union Research Center for Standardization and Certification of Raw Materials and Substances, USSR State Committee for Standards), 1987. Available from VNIIKI (All-Union Research Inst. of Space Studies), 1988, Moscow, no. 451.
Iomtev, M.B., Piskunov, V.G., Kadzhaev, V.L., and Gubanova, G.P.,Sistema gaz-lyod. Rastvorimost’ Vda v azote i argone v diapazone temperatur ot-40°C do -2°C i davlenii ot 0.2 MPa do 35.5 MPa. Tablitsy RSD-GSSSD R 178-86 (The Solubility of Ice in Nitrogen and Argon in the Range of Temperatures from -40°C to -2°C and Pressures from 0.2 MPa to 35.5 MPa: Tables of Recommended Reference Data-State Service of Standard and Reference Data R 178-86), Moscow: VNITs MV Gosstandarta SSSR (All-Union Research Center for Standardization and Certification of Raw Materials and Substances, USSR State Committee for Standards), 1986. Available from VNIIKI (All-Union Research Inst. of Space Studies), 1987, Moscow, no. 340.
Coan, C.R. and King, A.D.,J Am. Chem. Soc., 1971, vol. 93, no. 8, p. 1857.
Beketov, V.G., Rabinovich, V.A., and Rogovin, M.D.,Raschet termodinamicheskikh svoistv vlazhnykh gazov v oblasti parametrov ot 200 do 400 K i ot 0.1 do 10 MPa. Metodika GSSSD MR 99-93 (Analysis of the Thermodynamic Properties of Moist Gases in the Range of Parameters from 200 to 400 K and from 0.1 to 10 MPa: Procedure of the State Service of Standard and Reference Data MR 99-93), 1993. Available from VNITs SMV Gosstandarta Rossii (All-Russia Research Center for Standardization and Certification of Raw Materials and Substances, State Committee for Standards of the Russian Federation).
Beketov, V.G., Rabinovich, V.A., and Rogovin, M.D.,Teplofiz. Vys. Temp., 1994, vol. 32, no. 6, p. 829 (High Temp. (Engl. transl.), vol. 32, no. 6, p. 774).
Beketov, V.G. and Rabinovich, V.A.,Inzh. Fiz. Zh., 1993, vol. 64, no. 2, p. 172.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Beketov, V.G. Results of investigation of the solubility of ice and water in compressed gases. High Temp 38, 131–140 (2000). https://doi.org/10.1007/BF02755578
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02755578