Abstract
Objective
experimental studies have demonstrated that acute myocardial infarction (MI) alters energy metabolism even in non-infarcted adjacent tissue. In patients with subacute MI, the influence of the regional ischemie insult on energy metabolism of intact septal myocardium was analyzed using31P-Magnetic resonance spectroscopy (MRS).
Patients and Methods
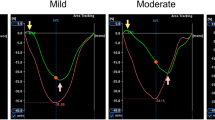
in eight patients with wall motion abnormalities in the anterior wall31P-spectra were obtained from non-infarcted adjacent scptal myocardium, as well as infarcted anterior myocardium (voxel size 25 ccm each) 29 ±8 days after MI using a 3D-CSI technique. Additionally, cardiac function was analyzed using breath-hold cine MRI. MR1 was repeated 6 months after revascularization to assess viability of infarcted segments. Eight age-matched healthy volunteers served as control group.
Results
according to follow-up MRI 4/8 patients showed regional wall motion recovery. Here, PCr/ATP-ratios were not significantly reduced in intact septal myocardium as well as infarcted anterior myocardium compared to healthy volunteers (1.28 ±0.10 and 1.14 ±0.09 vs. 1.45 ±0.29). No recovery of regional function was detected in 4/8 patients with —therefore—non-viable anterior myocardium. PCr/ATP-ratios were significantly reduced in intact and infarcted myocardium compared with healthy volunteers as well as to patients with wall motion recovery (0.77 ±0.17 and 0.49 ±0.23;P < 0.05).
Discussion
these preliminary results indicate that energy metabolism is reduced in patients with persisting wall motion abnormalities after myocardial infarction and revascularization in ischemically injured as well as in adjacent non-injured myocardium.
Similar content being viewed by others
References
Weiss RG, Kalil-Filho R, Herskowitz A, Chacko VP, Litt M, Stern MD, Gerstenblith G. Tricarboxylic acid cycle activity in postischemic rat hearts. Circulation 1993;87:270–82.
Neubauer S, Horn M, Naumann A, Tian R, Hu K, Laser M, Friedrich J, Gaudron P, Schnackerz K, Ingwall J, Ertl G. Impairment of energy metabolism in intact residual myocardium of rat hearts with chronic myocardial infarction. J Clin Invest 1995;95:1092–100.
Sanbe A, Tanonaka K, Hanaoka Y, Katoh T, Takeo. Regional energy metabolism of failing hearts following myocardial infarction. J Mol Cell Cardiol 1993;25:995–1013.
Sharkey SW, Murakami MM, Smith SA, Apple FS. Canine myocardial creatine kinase isoenzymes after chronic coronary artery occlusion. Circulation 1991;84:333–40.
Ingwall JS. Is cardiac failure a consequence of decreased energy reserve? Circulation 1993;87(Suppl 7):58–62.
Glasser SP. The time course of left ventricular remodeling after acute myocardial infarction. Am J Cardiol 1997;80:506–7.
Zhang J, Wilke N, Wang Y, Zhang Y, Wang C, Eijgelshoven MHJ, Cho YK, Murakami Y, Ugurbil K, Bache RJ, From AHL. Functional and bioenergetic consequences of postinfarc-tion left ventricular remodeling in a new porcine model: an MRI and31P MRS study. Circulation 1996;94:1089–99.
Neubauer S, Krahe T, Schindler R, Hillenbrand H, Entzeroth C, Horn M, Bauer WR, Stephan T, Lackner K, Haase A, Ertl G. Cardiac31P magnetic resonance spectroscopy of volunteers and patients with coronary artery disease and dilated cardiornyopa-thy. Altered cardiac high-energy phosphate metabolism in heart failure. Circulation 1992;86:1810–8.
Beer M, Sandstede J, Landschütz W, Viehrig M, Harre K, Horn M, Meininger M, Pabst T, Kenn W, Haase A. von Kienlin M, Neubauer S, Hahn D. Altered energy metabolism after myocardial infarction assessed by31P-MR-spectroscopy in humans. Eur Radiol 2000;10:1323–8.
Sandstede J, Lipke C, Beer M, Harre K, Pabst T, Kenn W, Neubauer S, Hahn D. Cine MRI for the evaluation of influences of regional left ventricular wall motion abnormalities on global cardiac function after myocardial infarction and revascularisa-tion therapy. Fortschr Roentgenstr 1999;171:424 30.
Sandstede JJW, Lipke C, Beer M, Harre K, Pabst T, Kenn W, Neubauer S, Hahn D. Analysis of first-pass and delayed contrast-enhancement patterns of dysfunctional myocardium on MR imaging: use in the prediction of myocardial viability. Am J Radiol 2000;174:1737–40.
Bottomley PA, Hardy CJ. Proton Overhauser enhancements in human cardiac phosphorus NMR spectroscopy at 1.5 T. Magn Reson Med 1992;24:384–90.
Vanhamme L, van den Boogaart A, van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson 1997;129:35–43.
Meininger M, Landschütz W, Beer M, Seyfarth T, Horn M, Pabst T, Haase A, Hahn D, Neubauer S, von Kienlin M. Concentrations of human cardiac phosphorus metabolites determined by SLOOP31P NMR spectroscopy. Magn Reson Med 1999;41:657–63.
Zhang J, Ugurbil K, From AHL, Bache RJ. Use of magnetic resonance spectroscopy for in vivo evaluation of high-energy phosphate metabolism in normal and abnormal myocardium. J Cardiovasc Magn Reson 2000;2(1):23–32.
Bolli R. Myocardial ‘stunning’ in man. Circulation 1992;86:1671–91.
Beer M, Landschütz W, Meininger M, Seyfarth T, Viehrig M, Sandstede J, Pabst T, Kenn W, Horn M, Harre K, van Kienlin M, Neubauer S, Hahn D. Determination of absolute concentrations of cardiac high-energy phosphates using31P-MR-spec-troscopy and SLOOP in healthy and diseased myocardium. Rofo Fortschr Geb Roentgenstr Neuen Bildgeb Verfahr 1999;171(1):65–8.
Bottomley PA. MR-spectroscopy of the human heart: the status and the challenges. Radiology 1994;191:593 612.
Kalil-Filho R, de Albuquerque CP, Weiss RG, Mocelim A, Belotti G, Cerri G, Pileggi F. Normal high energy phosphate reatios in ‘stunned’ human myocardium. J Am Coll Cardiol 1997;30(5): 1228–32.
Okada M, Mitsunami K, Inubushi T, Kinoshita M. Influence of aging or left ventricular hypertrophy on the human heart: contents of phosphorous metabolites measured by31P-MRS. Magn Reson Med 1998;39:772–82.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Beer, M., Buchner, S., Sandstede, J. et al. 31P-MR Spectroscopy for the evaluation of energy metabolism in intact residual myocardium after acute myocardial infarction in humans. MAGMA 13, 70–75 (2001). https://doi.org/10.1007/BF02668154
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02668154