Abstract
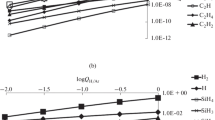
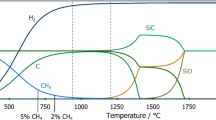
The carbothermic reduction of silicon dioxide at high temperature was studied using a free energy minimization (FEM) program. The stability boundaries of Si(l), SiC(s), and SiO2(l), and the equilibrium compositions of the gaseous species at various pressures, temperatures, and total composition of the system have been calculated based on available thermodynamic data. At 101.325 kPa total pressure, formation of Si(l) is possible if the initial C/SiO2 ratio in the system is kept between 1 and 2.34. Above this ratio, only SiC(s) is observed. The temperature range over which only Si(l) is present in the condensed phase depends on the composition and pressure of the system. For example, at an initial C/SiO2 ratio of 1.75 and 101.325 kPa pressure, Si(l) is stable from 3080 K to 2813 K, below which SiC(s) begins to form. The possibility of producing elemental silicon using carbothermic reduction of SiO2 in a plasma reactor is discussed.
Similar content being viewed by others
References
J. J. Moore, K. J. Reid, and J. K. Tylko:J. Met., 1981, vol. 33(8), pp. 43–49.
C. B. Alcock:Pure Appl. Chem., 1980, vol. 52, pp. 1817–27.
J. K. Tylko, J. J. Moore, and K. J. Reid:Proceedings of Extraction Metallurgy ’81, International Conference of the Institute of Mining and Metallurgy, London, England, Sept. 1981, pp. 377–417.
L. A. Ettlinger, T. D. Nainan, R. P. Ouellette, and P. N. Cheremisinoff:High-Temperature Plasma Technology Applications, Ann Arbor Science Publishers, Inc., Ann Arbor, MI, 1980, ch. 1–2.
L. M. Naphtali:Ind. Eng. Chem., 1961, vol. 53(5), pp. 387–88.
R. C. Oliver, S. E. Stephanou, and R.W. Baier:Chem. Eng., 1962, Feb. 19, pp. 121–28.
R. G. Anthony and D. M. Himmelblau:J. Phys. Chem., 1963, vol. 67, pp. 1080–83.
B. George, L. P. Brown, C. H. Farmer, P. Buthod, and F. S. Manning:Ind. Eng. Chem., Process Des. Dev., 1976, vol. 15, pp. 372–77.
A. M. Kuhlmann: U. S. Patent 3, 215, 522, 1965.
W. T. Fairchild:J. Met., 1970, vol. 22(8), pp. 55–58.
A. Gosh and G. R. St. Pierre:Trans. TMS-AIME, 1969, vol. 245, pp. 2106–08.
W. A. Krivsky and R. Schuhmann, Jr.:Trans. TMS-AIME, 1961, vol. 221, pp. 898–904.
G. Eriksson and T. Johansson:Scand. J. Metall., 1978, vol. 7, pp. 264–70.
G. Eriksson and T. Johansson:Scand. J. Metall., 1980, vol. 9, pp. 283–91.
T. Johansson and G. Eriksson:J. Electrochem. Soc., 1984, vol. 131, pp. 365–70.
E. T. Turkdogan, G. J. W. Kor, and R. J. Fruehan:Ironmaking Steel-making, 1980, no. 6, pp. 268–80.
M. Nagamori, I. Malinsky, and A. Claveau:Metall. Trans. B, 1986, vol. 17B, pp. 503–14.
T. Rosenqvist and J. K. R. Tuset:Metall. Trans. B, 1987, vol. 18B, pp. 471–72.
M. Nagamori, I. Malinsky, and A. Claveau:Metall. Trans. B, 1987, vol. 18B, pp. 472–77.
E. K. Stanley:Electr. Furn. Conf. Proc., 1984, vol. 42, pp. 151–55.
S. Gordon and B. J. McBride: NASA SP273, NASA-Lewis, Cleveland, OH, 1971.
JANAF Thermochemical Tables: U.S. Government Doc. No. NSRDS-NBS 37, 2nd ed., 1971; supplements inJ. Phys. Chem. Ref. Data: 1974, vol.’ 3(2); 1975, vol. 4(1); 1978, vol. 7(3); 1982, vol. 11(3).
O. Kubaschewski and C. B. Alcock:Metallurgical Thermochemistry, 5th ed., Pergamon Press, New York, NY, 1979, pp. 221–25.
A. Schei and K. Larsen:Electr. Furn. Conf. Proc., 1981 (Pub. 1982), vol. 39, pp. 301–09.
R. Hultgren, P. D. Desai, D. T. Hawkins, M. Gleiser, and K. K. Kelley:Selected Values of the Thermodynamic Properties of Binary Alloys, ASM, Metals Park, OH, 1973, p. 879.
J. A. Batdorf, B. A. Detering, and C. M. Wai: EG&G Idaho, Inc., Idaho Falls, ID, and University of Idaho, Moscow, ID, unpublished research, 1987.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hutchison, S.G., Richardson, L.S. & Wai, C.M. Carbothermic reduction of silicon dioxide— a thermodynamic investigation. Metall Trans B 19, 249–253 (1988). https://doi.org/10.1007/BF02654209
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02654209