Abstract
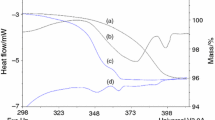
Nafagrel hydrochloride has two kinds of stable pseudopolymorphs such as hemihydrate and monohydrate. The dehydration of crystal water of these hydrates took place in one step under nitrogen gas atmosphere, whereas the two dehydration steps could be detected for the monohydrate under self-generated atmosphere such as the quasi-sealed and/or the completely sealed systems. These observations indicated that the crystal water of the monohydrate consisted of two different crystal waters.
Prediction of the stability for the hydrates using the kinetic parameters indicated that the dehydration of the monohydrate occurred faster than that of the hemihydrate.
Zusammenfassung
Nafagrel Hydrochlorid besitzt zwei Arten von stabilen pseudopolymorphen Erscheinungsformen: Halbhydrat und Monohydrat. Die Dehydratation des Kristallwassers dieser Komplexe erfolgt in Stickstoffatmosphäre in zwei Schritten, während für das Monohydrat in selbstgenerierter Atmosphäre (in halb- oder vollständig geschlossenem System) zwei Dehydratationsschritte beobachtet werden konnten. Diese Beobachtungen zeigen, daß das Kristallwasser des Monohydrates aus zwei verschiedenen Arten von Kristallwasser besteht.
Eine Voraussage der Stabilität der Hydrate unter Anwendung der kinetischen Parameter zeigt, daß die Dehydratation des Monohydrates schneller verläuft als die des Halbhydrates.
Similar content being viewed by others
References
M. Kanao, Y. Watanabe, Y. Kimura, J. Saegusa, K. Yamamoto, H. Kanno, N. Kanaya, H. Kubo, S. Ashida and F. Ishikawa, J. Med. Chem., 32 (1989) 1326.
K. Isa and H. Okuno, Bull. Chem. Soc. Japan, 55 (1982) 3733.
T. Ozawa, Bull. Chem. Soc. Jpn., 38 (1965) 1881.
T. Ozawa, Bull. Chem. Soc. Jpn., 57 (1984) 639.
J. H. Sharp, G. W. Brindley and B. N. N. Achar, J. Am. Ceram. Soc., 49 (1966) 379.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kitaoka, H., Ohya, K. The dehydration behavior of nafagrel hydrochloride hydrates. Journal of Thermal Analysis 40, 387–394 (1993). https://doi.org/10.1007/BF02546606
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02546606