Abstract
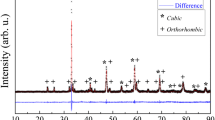
A SOFC cathode related perovskite material, (La0.7Sr0.3)0.9MnO3, has been investigated by simultaneous thermogravimetry - mass spectrometry from room temperature to 1770 K. Water, carbon dioxide and oxygen were detected by mass spectrometry. Water and carbon dioxide evolution can be interpreted by assuming that prior to the thermogravimetry-mass spectrometry measurement about 0.5 % of the lanthanum component had reacted with carbon dioxide and water to form La2(CO3)3*8H2O, which dehydrated and decomposed via La2O2CO3 into La2O3 and evolving H2O and CO2 during the present experiment. The observation that the lanthanum strontium manganite emitted oxygen in two stages can be ascribed to the two different oxygen sites in the perovskite lattice, that is, the oxygen excess and deficient regions.
Similar content being viewed by others
References
M. Dokiya, N. Sakai, T. Kawada, H. Yokokawa, T. Iwata, and M. Mori, in: Proceedings of the First International Symposium on Solid Oxide Fuel Cells (S. C. Singhal Ed.), PV 89–11, The Electrochemical Society Proceedings series, Pennington, NJ, 1989, p. 325.
H. Yokokawa, N. Sakai, T. Kawada, M. Dokiya, in: Proceedings of international conference on SOFC Nagoya (M. Dokiya Ed.), Science House, Tokyo, 1990, p. 118.
H. Yokokawa, N. Sakai, T. Kawada, M. Dokiya, Denki Kagaku57, 829 (1989).
H. Yokokawa, N. Sakai, T. Kawada, M. Dokiya, J. Electrochem. Soc.138, 2719 (1991).
E. Bergsmark, S. Furuseth, O. Dyrlie, T. Norby and P. Kofstad, in: Proceedings of the Second International Symposium on Solid Oxide Fuel Cells (F. Grosz, P. Zegers, S. C. Singhal, and O. Yamamoto Ed.), Commission of the European Communities, 1991, p. 473.
B. C. H. Steele, S. Carter, J. Kajda, I. Kortoulis and J. A. Kilner, in: Proceedings of the Second International Symposium on Solid Oxide Fuel Cells (F. Grosz, P. Zegers, S. C. Singhal, and O. Yamamoto Ed.), Commission of the European Communities, 1991, p. 517.
Y. Takai, K. Ishikiriyama, and M. Todoki, in: Proceedings of the 27th Calorimetric Conference of Japan, The Japan Society of Calorimetry and Thermal Analysis, Tokyo, 1991, p. 52.
T. Wada, H. Yamauchi and S. Tanaka, J. Am. Ceram. Soc.75, 1705 (1992).
M. Akinc and D. Sordelet, Advance Ceram. Mater.2, 232 (1987).
R. L. N. Sastry, S. R. Yoganarasimhan, P. N. Mehrotra and C. N. R. Rao, J. Ionorg. Nucl. Chem.28, 1165 (1966).
H. Hinode, R. Sharma and L. Eyring, J. Solid State Chem.84, 102 (1990).
L. Moscardini D'Assuncào, I. Giolito and M. Ionashiro, Thermochimica Acta137, 319 (1989).
H. Bergmann ed. Gmelin Handbook of Inorganic and Organometallic Chemistry, 8th edition, Rare earth elements C12b Compounds with carbon, 1994, pp. 27–30.
H. Yokokawa, N. Sakai, T. Kawada, and M. Dokiya, Denki Kagaku58, 561 (1990).
H. Yokokawa, N. Sakai, T. Kawada, and M. Dokiya, Denki Kagaku58, 341 (1990).
J. Mizusaki and H. Tagawa, Solid State Ionics49, 111 (1991).
A. Belzner, T. M. Gür, and R. A. Huggins, Solid State Ionics57, 327 (1992).
H. Yokokawa, T. Kawada, and M. Dokiya, J. Am. Ceram. Soc.72, 2104 (1989).
H. Yokokawa, N. Sakai, T. Kawada, and M. Dokiya, J. Electrochem. Soc.137, 388 (1990).
H. Yokokawa, N. Sakai, T. Kawada, M. Dokiya, J. Am. Ceram. Soc.73, 649 (1990).
D. D. Wagman, W. H. Evans, V. B. Parker, R. H. Schumm, I. Halow, S. M. Bailey, K. L. Churney, R. L. Nuttall, The NBS tables of chemical thermodynamic properties, J. Phys. Chem. Ref. Data11, supplement No. 2, 1982.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yokokawa, H., Sakai, N., Horita, T. et al. Simultaneous thermogravimetry - mass spectrometry for a solid oxide fuel cell cathode: (La0.7Sr0.3)0.9MnO3 . Ionics 2, 190–195 (1996). https://doi.org/10.1007/BF02376020
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02376020