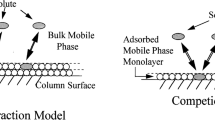
Summary
The linear Jaroniec equation has been utilized for the description of chromatographic systems in which the test substance molecule is solvated by the solvent molecules. A possibility of the formation of multimolecular solvates in the bulk phase has been confirmed. The measurements were made using liquid adsorption chromatography with ternary mobile phase.
Similar content being viewed by others
Abbreviations
- C1 :
-
equilibrium constant of the formation of an s−1 type bimolecular solvate
- C2 :
-
equilibrium constant of the formation of solvates containing 1+z molecules
- C *1 :
-
equilibrium constant corresponding to the mean energy of bonding the molecule of the most polar solvent to the test substance molecule
- k ′s :
-
capacity factor of the chromatographed substance
- Ks1 :
-
phase exchange equilibrium constant of the chromatographed substance
- K *s1 :
-
equilibrium constant of phase exchange considering the existence of multimolecular solvates of the substance
- L2 :
-
equilibrium constant of the formation of 1–1 type double associates of the most polar mobile phase component
- q:
-
a proportionality coefficient characteristic of a given adsorbent and independent on the nature of the mobile phase
- Qz :
-
molar fraction of 1+z molecular solvates in the mobile phase
- r:
-
ratio of the surface area occupied by a test substance molecule to the surface area of a molecule of component 1 of the mobile phase
- x1 :
-
total molar fraction of the most polar component of the mobile phase
- x 0s :
-
total molar fraction of the test substance in the mobile phase
- xs :
-
molar fraction of the test substance in the mobile phase
- y1 :
-
molar fraction of solvent 1 in the surface phase
- ys :
-
molar fraction of the test substance in the surface phase
- z:
-
the number of solvent molecules in a 1+z solvate
References
L. R. Snyder, “Principles of Adsorption Chromatography”, Dekker, New York, 1968
J. Ościk, Przem. Chem.,44, 3 (1965)
M. Jaroniec, J. Ościk, J. HRC & CC,5, 3 (1982)
E. Soczewiński, Anal. Chem.,41, 179 (1969)
M. Jaroniec, J. A. Jaroniec, J. Liquid Chromatogr.,7, 393 (1984)
J. K. Róźyŀo, M. Jaroniec, J. A. Jaroniec, J. Liquid Chromatogr.,8, 651 (1985)
M. Jaroniec, J. A. Jaroniec, J. Liquid Chromatogr.,4, 2121 (1981)
M. Jaroniec, J. A. Jaroniec, J. Chromatogr.,210, 130 (1981)
M. Jaroniec, J. A. Jaroniec, W. Golkiewicz, J. HRC & CC,4, 89 (1981)
M. Jaroniec, J. K. Róźyŀo, J. A. Jaroniec, Chem. Anal.26, 623 (1981)
B. Ościk-Mendyk, J. K. Róźyŀo, Chromatographia,25, 300 (1988)
B. Ościk-Mendyk, J. K. Róźyŀo, J. A. Jaroniec, J. Liquid Chromatogr.,10, 2845 (1987)
M. Jaroniec, J. A. Jaroniec, J. Chromatogr.,295, 377 (1984)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ościk-Mendyk, B. Molecular interactions in liquid adsorption chromatography with mixed mobile phase. Solvation of the chromatographed substance in a ternary mobile phase. Chromatographia 28, 151–156 (1989). https://doi.org/10.1007/BF02319638
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02319638