Abstract
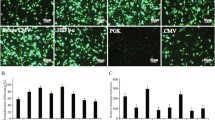
We have explored the possibility of using avian cells for the expression of human proteins. We found that various avian cells including quail fibrosarcoma cells (QF), duck embryo cells (DE) and primary chicken embryo fibroblasts (CE) could efficiently be transfected with DNA by calcium phosphate coprecipitation, and that promoters which are transcriptionally active in mammalian cells also functioned well in these avian cells. Among the promoters we tested, the major immediate early promoter of human cytomegalovirus drove the highest level of chloramphenicol acetyl transferase (CAT) expression, outperforming the SV40 early promoter and the RSV LTR. Using the bacterial CAT gene as a reporter, we found that levels of CAT activity were higher in QF and DE cells than in mammalian cells such as CHO, HeLa, Vero and 293T cells. We further cloned a sequence encoding human erythropoietin (EPO) and compared its expression in QF and mammalian cells. Consistent with the CAT data, in transient transfection assays, QF cells produced higher levels of EPO than the mammalian cell lines tested. QF cells which can be passaged permanently were stably transfected with an EPO expression vector. The subcloned QF line was able to produce up to 1,700 U/ml EPO from 3 × 106 cells in 72 h. Purified QF-produced EPO showed a broad but discrete protein band, ranging from 33 to 41 kD and was as biologically active as CHO-produced EPO. Although a number of factors still remain to be optimized, our results demonstrate the potential of avian cells such as QF as producers of heterologous proteins.
Similar content being viewed by others
References
Broudy VC, Tait JF, Powell JS. Recombinant human erythropoietin: Purification and analysis of carbohydrate linkage. Arch Biochem Biophys 2:329–336;1988.
Burnet FM. Principles of Animal Virology. New York, Academic Press, 1960
Corsaro CM, Pearson ML. Enhancing the efficiency of DNA-mediated gene transfer in mammalian cells. Somat Cell Genet 7:603–616;1981.
Dordal MS, Wang FF, Goldwasser E. The role of carbohydrate in erythropoietin action. Endocrinology 116:2293–2299;1985.
Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan R. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA 90:3539–3543;1993.
Dube S, Fisher JW, Powell JS. Glycosylation at specific sites of erythropoietin is essential for biosynthesis, secretion, and biological function. J Biol Chem 263:17516–17521;1988.
DuBridge RB, Tang P, Hsia HC, Leong DM, Miller JH, Calos MP. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol Cell Biol 7:379–387;1987.
Fenner F, McAuslan BR, Mims CA, Sambrook J, White DO. The Biology of Animal Viruses. New York, Academic Press, 1974.
Goldberg MA, Glass GA, Cunningham JM, Bunn HF. The regulated expression of erythropoietin by two human hepatoma cell lines. Proc Natl Acad Sci USA 84:7972–7976;1987.
Gorman CM, Merlino GT, Willingham MC, Pastan I, Howard BH The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci USA 79:6777–6781;1982.
Hayward BE, Hussain A, Wilson RH, Lyons A, Woodcock V, McIntosh B, Harris TJ. The cloning and nucleotide sequence of cDNA for an amplified glutamine synthetase gene from the Chinese hamster. Nucleic Acids Res 14:999–1008;1986.
Hirst GK. In Horsefall FL, Tamm I (eds). Viral and Rickettsial Diseases of Man. Philadelphia, Lippincott, p 216;1965.
Jacobs K, Shoemaker C, Rudersdorf R, Neill SD, Kaufman RJ, Mufson A, Seehra J, Jones SS, Hewick R, Fritsch EF, Kawakita M, Shimizu T, Miyake T. Isolation and characterization of genomic and cDNA clones of human erythropoietin. Nature 313:806–810;1985.
Kim S, Ikenchi K, Groopman J, Baltimore D. Factors affecting cellular tropism of human immunodeficiency viruses. J Virol 64:5600–5604;1990.
Krystal G. A simple microassay for erythropoietin based3H-thymidine incorporation into spleen cells from phenylhydrazine treated mice. Exp Hematol 11:649–660;1983.
Lin F, Suggs S, Lin C, Browne JK, Smalling R, Egrie JC, Chen KK, Fox GM, Martin F, Stabinsky Z, Badrawi SM, Lai P, Goldwasser E. Cloning and expression of the human erythropoietin gene. Proc Natl Acad Sci USA 82:7580–7584;1985.
Pear WS, Nolan GP, Scott ML, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA 90:8392–8396;1993.
Rous PA. A sarcoma of the fowl transmissible by an agent separable from the tumor cell. J Exp Med 13:397–411;1911.
Sasaki H, Bothner B, Dell A, Fukuda M. Carbohydrate structure of erythropoietin expressed in Chinese hamster ovary cells by a human erythropoietin cDNA. J Biol Chem 262:12059–12076;1987.
Stehelin D, Guntaka RV, Varmus HE, Bishop JM. Purification of DNA complementary to nucleotide sequences required for neoplastic transformation of fibroblasts by avian sarcoma virus. J Mol Biol 101:349–365;1976.
Stehelin D, Varmus HE, Bishop JM, Vogt PK. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature 260:170–173;1976.
Thomsen DR, Sternberg RM, Goins WF, Stinski MF. Promoter regulatory region of the major immediate early gene of human cytomegalovirus. Proc Natl Acad Sci USA 81:659–663;1984.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lee, SY., Kim, S.H., Kim, V.N. et al. Heterologous gene expression in avian cells: Potential as a producer of recombinant proteins. J Biomed Sci 6, 8–17 (1999). https://doi.org/10.1007/BF02256418
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02256418