Abstract
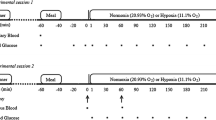
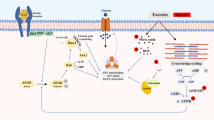
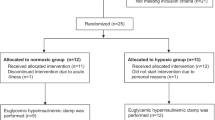
We compared the chronic effect of intermittent hypoxia and endurance training on the glucose tolerance and GLUT4 protein expression in rat skeletal muscle. Thirty-two Sprague-Dawley rats were matched for weight and assigned to one of the following four groups: control, endurance training, hypoxia, or hypoxia followed by endurance training. Hypoxic treatment consisted of breathing 14% O2 for 12 h/day under normobaric conditions, and the training protocol consisted of making animals swim 2 times for 3 h/day. At the end of the 3rd week, an oral glucose tolerance test (OGTT) was performed 16 h after treatments. At the end of the 4th week, GLUT4 protein, mRNA, and glycogen storage in skeletal muscle were determined. Endurance training significantly improved OGTT results. Glycogen content and GLUT4 protein expression in the plantaris and red gastrocnemius, but not in the soleus or white gastrocnemius muscles, were also elevated. Chronic intermittent hypoxia also improved OGTT results, but did not alter GLUT4 protein expression. Additionally, hypoxia followed by exercise training produced significant increases in GLUT4 protein and mRNA in a greater number of muscles compared to endurance training alone. Both exercise training and hypoxia significantly reduced body mass, and an additive effect of both treatments was found. In conclusion, chronic intermittent hypoxia improved glucose tolerance in the absence of increased GLUT4 protein expression. This treatment facilitated the exercise training effect on muscle GLUT4 expression and glycogen storage. These new findings open the possibility of utilizing intermittent hypoxia, with or without exercise training, for the prevention and clinical treatment of type 2 diabetes or insulin resistance.
Similar content being viewed by others
References
Amsel J, Waterbor JW, Oler J, Rosenwaike I, Marshall K. Relationship of site-specific cancer mortality rates to altitude. Carcinogenesis 3:461–465;1982.
Baron AD, Steinberg H, Brechtel G, Johnson A. Skeletal muscle blood flow independently modulates insulin-mediated glucose uptake. Am J Physiol 266:E248-E253;1994.
Baron AD, Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G. Insulin-mediated skeletal muscle vasodilation contributes to both insulin sensitivity and responsiveness in lean humans. J Clin Invest 96:786–792;1995.
Baron AD, Tarshoby M, Hook G, Lazaridis EN, Cronin J, Johnson A, Steinberg HO. Interaction between insulin sensitivity and muscle perfusion on glucose uptake in human skeletal muscle: evidence for capillary recruitment. Diabetes 49:768–774;2000.
Birnbaum MJ. Diabetes. Dialogue between muscle and fat. Nature 409:672–673;2001.
Brozinick JT Jr, Etgen GJ Jr, Yaspelkis BB 3rd, Kang HY, Ivy JL. Effects of exercise training on muscle GLUT-4 protein content and translocation in obese Zucker rats. Am J Physiol 265:E419-E427;1993.
Bruckner BA, Ammini CV, Otal MP, Raizada MK, Stacpoole PW. Regulation of brain glucose transporters by glucose and oxygen deprivation. Metabolism 48:422–431;1999.
DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 30:1000–1007;1981.
Delbono O. Neural control of aging skeletal muscle (review). Aging Cell 2:21–29;2003.
Deveci D, Marshall JM, Egginton S. Relationship between capillary angiogenesis, fiber type, and fiber size in chronic systemic hypoxia. Am J Physiol 281:H241-H252;2001.
Dill RP, Chadan SG, Li C, Parkhouse WS. Aging and glucose transporter plasticity in response to hypobaric hypoxia. Mech Ageing Dev 122:533–545;2001.
Dohm GL, Sinha MK, Caro JF. Insulin receptor binding and protein kinase activity in muscles of trained rats. Am J Physiol 252:E170-E175;1987.
Evans DJ, Murray R, Kissebah AH. Relationship between skeletal muscle insulin resistance, insulin-mediated glucose disposal, and insulin binding. Effects of obesity and body fat topography. J Clin Invest 74:1515–1525;1984.
Facchini FS, Hua N, Abbasi F, Reaven GM. Insulin resistance as a predictor of age-related diseases. J Clin Endocrinol Metab 86:3574–3578;2001.
Fushiki T, Kano T, Ito K, Hirofuji C, Inoue K, Moritani T, Sugimoto E. Effects of chronic hypoxia on the whole-body insulin action in rats. Can J Physiol Pharmacol 70:1522–1524;1992.
Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol 88:2187–2195;2002.
Ivy JL, Zderic TW, Fogt DL. Prevention and treatment of non-insulin-dependent diabetes mellitus. Exerc Sport Sci Rev 27:1–35;1999.
Joost HG, Thorens B. The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members (review). Mol Membr Biol 18:247–256;2001.
Kern M, Wells JA, Stephens JM, Elton CW, Friedman JE, Tapscott EB, Pekala PH, Dohm GL. Insulin responsiveness in skeletal muscle is determined by glucose transporter (Glut4) protein level. Biochem J 270:397–400;1990.
Kuo CH, Browning KS, Ivy JL. Regulation of GLUT4 protein expression and glycogen storage after prolonged exercise. Acta Physiol Scand 165:193–201;1999.
LaManna JC, Kuo NT, Lust WD. Hypoxia-induced brain angiogenesis. Signals and consequences. Adv Exp Med Biol 454:287–293;1998.
Larsen JJ, Hansen JM, Olsen NV, Galbo H, Dela F. The effect of altitude hypoxia on glucose homeostasis in men. J Physiol 504:241–249;1997.
MacLean PS, Zheng D, Dohm GL. Muscle glucose transporter (GLUT 4) gene expression during exercise. Exerc Sport Sci Rev 28:148–152;2000.
Megeney LA, Prasad MA, Tan MH, Bonen A. Expression of the insulin-regulatable transporter GLUT-4 in muscle is influenced by neurogenic factors. Am J Physiol 266:E813-E816;1994.
Mortimer EAJr, Monson RR, MacMahon B. Reduction in mortality from coronary heart disease in men residing at high altitude. N Engl J Med 296:581–585;1977.
Mu J, Brozinick JTJr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell 7:1085–1094;2001.
Paulsen SR, Rubink DS, Winder WW. AMP-activated protein kinase activation prevents denervation-induced decline in gastrocnemius GLUT-4. J Appl Physiol 91:2102–2108;2001.
Randle PJ, Smith GH. Regulation of glucose uptake by muscle. The effects of insulin, anaerobiosis and cell poisons on uptake of glucose and release of potassium by isolated rat diaphragm. Biochem J 70:490–508;1958.
Rattigan S, Clark MG, Barrett EJ. Hemodynamic actions of insulin in rat skeletal muscle: evidence for capillary recruitment. Diabetes 46:1381–1388;1997.
Ray PS, Estrada-Hernandez T, Sasaki H, Zhu L, Maulik N. Early effects of hypoxia/reoxygenation on VEGF, ang-1, ang-2 and their receptors in the rat myocardium: implications for myocardial angiogenesis. Mol Cell Biochem 213:145–153;2000.
Ryder JW, Yang J, Galuska D, Rincón J, Björnholm M, Krook A, Lund S, Pedersen O, Wallberg-Henriksson H, Zierath JR, Holman GD. Use of a novel impermeable biotinylated photolabeling reagent to assess insulin- and hypoxia-stimulated cell surface GLUT4 content in skeletal muscle from type 2 diabetic patients. Diabetes 49:647–654;2000.
Tsao TS, Stenbit AE, Factor SM, Chen W, Rossetti L, Charron MJ. Prevention of insulin resistance and diabetes in mice heterozygous for GLUT4 ablation by transgenic complementation of GLUT4 in skeletal muscle. Diabetes 48:775–782;1999.
Vincent KA, Feron O, Kelly RA. Harnessing the response to tissue hypoxia: HIF-1 alpha and therapeutic angiogenesis. Trends Cardiovasc Med 12:362–367;2002.
Wagner PD. Skeletal muscle angiogenesis. A possible role for hypoxia. Adv Exp Med Biol 502, 21–38;2001.
Winder WW. Energy-sensing and signaling by AMP-activated protein kinase in skeletal muscle. J Appl Physiol 91:1017–1028;2001.
Xia Y, Warshaw JB, Haddad GG. Effect of prolonged intermittent hypoxia on glucose transporters in heart and skeletal muscle of immature and adult rats. Am J Physiol 273:R1734-R1741;1997.
Yaspelkis BB 3rd, Castle AL, Farrar RP, Ivy JL. Effect of chronic electrical stimulation and beta-GPA diet on GLUT4 protein concentration in rat skeletal muscle. Acta Physiol Scand 163:251–259;1998.
Zhong Y, Heather LM, Randall SJ, Amato JG. Inhibition of PPARγ2 gene expression by the HIF-1 regulated gene DEC.Stra13. A mechanism for regulation of adipogenesis by hypoxia. Dev Cell 2:331–341;2002.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chiu, LL., Chou, SW., Cho, YM. et al. Effect of prolonged intermittent hypoxia and exercise training on glucose tolerance and muscle GLUT4 protein expression in rats. J Biomed Sci 11, 838–846 (2004). https://doi.org/10.1007/BF02254369
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02254369