Abstract
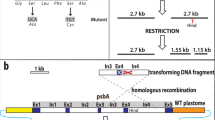
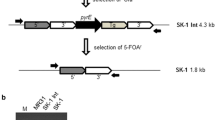
In this paper, we describe a protocol to obtain a site-directed mutants in thepsbA gene ofChlamydomonas reinhardtii, which overcomes several drawbacks of previous protocols, and makes it possible to generate a mutant within a month. Since the large size of the gene, and the presence of four large introns has made molecular genetics of thepsbA gene rather unwieldy, we have spliced all of the exons of thepsbA gene by PCR to facilitate genetic manipulation and sequencing of the gene. The resultant construct (plasmid pBA153, with several unique restriction sites introduced at exon boundaries) carried 1.2 and 1.8 kb intact sequences from the 5′- and 3′-flanking regions, respectively. The plasmid was used to transform a D1-deletion mutant and was found to complement the deletion and restore photosynthetic activity. In addition, a bacterialaadA gene conferring spectinomycin resistance (spe r) was inserted downstream of the intron-freepsbA gene, to give construct pBA155. This allowed selection of mutant strains deficient in photosynthesis by using spectinomycin resistance, and eliminated the possibility of selection for revertant strains which is a consequence of having to use photosynthetic activity as a selection pressure. Finally, pBA155 was used to construct pBA157, in which additional restriction sites were inserted to facilitate cassette mutagenesis for generation of mutations in spans thought to be involved in donor-side interactions. AllpsbA deletion strains transformed with intron-freepsbA-aadA constructs encoding the wild-type D1 sequence, and screened on spectinomycin plates for thespe r phenotype, were able to grow photosynthetically, and all showed identical kinetics for electron transfer from primary (QA) to secondary quinone (QB) in Photosystem II, as assayed by the decay of the high fluorescence yield on oxidation of the reduced primary acceptor (QA −).
Similar content being viewed by others
Abbreviations
- C. reinhardtii :
-
Chlamydomonas reinhardtii
- DCMU:
-
3-(3,4-dichlorophenyl)-1,1-dimethylurea
- E. coli :
-
Escherichia coli
- F0 :
-
non-variable fluorescence yield
- Fmax :
-
maximal fluorescence yield
- Fv :
-
variable fluorescence yield
- HS:
-
high-salt
- spe r :
-
spectinomycin resistance
- TAP:
-
Tris-acetate-phosphate
References
Blowers AD, Bogorad L, Shark KB and Sanford JC (1989) Studies onChlamydomonas chloroplast transformation foreign DNA can be stably maintained in the chromosome. Plant Cell 1: 123–132
Boynton JE, Gillham NW, Harris EH, Hosler JP, Johnson AM, Jones AR, Randolph-Anderson BL, Robertson D, Klein TM, Shark KB and Sanford JC (1988) Chloroplast transformation inChlamydomonas with high velocity microprojectiles. Science 240: 1534–1538
Crofts AR, Baroli I, Kramer D and Taoka S (1992) Kinetics of electron transfer between Qa and Qb in wild type and herbicide-resistant mutants ofChlamydomonas reinhardtii. Z Naturforsch 48c: 259–266
Debus RJ (1992) The manganese and calcium ions of photosynthetic oxygen evolution. Biochim Biophys Acta 1102: 269–352
Erickson JM, Rahire M and Rochaix JD (1984)Chlamydomonas reinhardii gene for the 32000-molecular-weight protein of Photosystem II contains 4 large introns and is located entirely within the chloroplast inverted repeat. EMBO J 3: 2753–2762
Erickson JM, Rahire M, Rochaix JD and Mets L (1985a) Herbicide resistance and cross-resistance changes at 3 distinct sites in the herbicide-binding protein. Science 228: 204–207
Erickson JM, Rochaix JD and Delepelaire P (1985b) Analysis of genes encoding two Photosystem II proteins of the 30–34-kilodalton size class. In: Steinback B, Boniz S, Arntzen CJ and Bogorad L (eds) Molecular Biology of the Photosynthetic Apparatus; Nato Advanced Research Workshop, pp 53–65. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Erickson JM, Whitelegge J, Koo D and Boyd K (1992) Site-directed mutagenesis of the Photosystem II D1 gene and transformation of theChlamydomonas chloroplast genome. In: Murata N (ed) Research in Photosynthesis, pp 421–424. Kluwer Academic Publishers, Dordrecht, The Netherlands
Finer JJ, Vain P, Jones MW and McMullen MD (1992) Development of the particle inflow gun for DNA delivery to plant cells. Plant Cell Reports 11: 323–328
Gillham NW (1978) Organelle Heredity, pp 347–380. Raven Press, New York, New York
Goldschmidt-Clermont M (1991) Transgenic expression of aminoglycoside adenine transferase in the chloroplast: A selectable marker for site-directed transformation ofChlamydomonas. Nucleic Acids Res 19: 4083–4089
Gorman DS and Levine RP (1965) Cytochrlmef and plastocyanin: Their sequence in the photosynthetic electron transport chain ofChlamydomonas reinhardi. Proc Natl Acad Sci USA 54: 1665–1669
Hanahan D (1985) Techniques for transformation ofE. coli. In: Glover DM (ed) DNA Cloning, pp 109–135. IRL Press, Oxford, UK
Heiss S and Johanningmeier U (1992) Analysis of a herbicide resistant mutant obtained by transformation of theChlamydomonas chloroplast. Photosynth Res 34: 311–317
Herrin DL, Bao Y, Thompson AJ and Chen Y-F (1991) Self-splicing of theChlamydomonas chloroplastpsbA introns. Plant Cell 3: 1095–1107
Johanningmeier U and Heiss S (1993) Construction of aChlamydomonas-reinhardtii mutant with an intronlesspsbA gene. Plant Mol Biol 22: 91–99
Joliot P, Bennoun P and Joliot A (1973) New evidence supporting energy transfer between photosynthetic units. Biochim Biophys Acta 305: 317–328
Keller M and Stutz E (1984) Structure of the Euglena gracilis chloroplast gene (psbA) coding for the 32-kDa protein of photosystem II. FEBS lett 175: 173–177
Kramer DM, Robinson HR and Crofts AR (1990) A portable multiflash kinetic fluorimeter for measurement of donor and acceptor reactions of Photosystem 2 in leaves of intact plants under field conditions. Photosynth Res 26: 181–194
Kück U, Choquet Y, Schneider M, Dron M and Bennoun P (1987) Structural and transcription analysis of two homologous genes for the P700 chlorophylla-apoproteins inChlamydomonas reinhardii: Evidence for in vivo trans-splicing. EMBO J 6: 2185–2195
Lers A, Heifetz PB, Boynton JE, Gillham NW and Osmond CB (1992) The carboxyl-terminal extension of the D1 protein of photosystem II is not required for optimal photosynthetic performance under CO2-and light-saturated growth conditions. J Biol Chem 267: 17494–17497
Myers AM, Grant DM, Rabert DK, Harris EH, Boynton JE and Gillham NW (1982) Mutants ofChlamydomonas reinhardtii with physical alterations in their chloroplast DNA. Plasmid 7: 133–151
Palmer JD, Boynton JE, Gillham NW and Harris EH (1985) Evolution and recombination of the large inverted repeat in chlamydomonas chloroplast DNA. In: Steinback KE, Bonitz S, Arntzen CK and Bogorad L (eds) Molecular Biology of the Photosynthetic Apparatus, pp 269–278. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Rochaix J-D (1987) Molecular genetics of chloroplasts and mitochondria in the unicellular green algaChlamydomonas. FEMS Microbiol Rev 46: 13–34
Rochaix J-D and Malnoe P (1978) Anatomy of the chloroplast ribosomal DNA ofChlamydomonas reinhardii. Cell 15: 661–670
Roffey RA, Golbeck JH, Hille CR and Sayre RT (1991) Photosynthetic electron transport in genetically altered Photosystem II reaction centers of chloroplasts. Proc Natl Acad Sci USA 88: 9122–9126
Sambrook J, Fritsch EF and Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Sanger F, Nicklen S and Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467
Sueoka N (1960) Mitotic replication of deoxyribonucleic acid inChlamydomonas reinhardi. Proc Natl Acad Sci USA 46: 83–91
Svab Z and Maliga P (1993) High-frequency plastid transformation in tobacco by selection for a chimericaadA gene. Proc Natl Acad Sci USA 90: 913–917
Turmel M, Boulanger J and Lemieux C (1989) Two group I introns with long internal open reading frames in the chloroplastpsbA gene ofChlamydomonas moewusii. Nucleic Acids Res 17: 3875–3886
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Minagawa, J., Crofts, A.R. A robust protocol for site-directed mutagenesis of the D1 protein inChlamydomonas reinhardtii: A PCR-splicedpsbA gene in a plasmid conferring spectinomycin resistance was introduced into apsbA deletion strain. Photosynth Res 42, 121–131 (1994). https://doi.org/10.1007/BF02187123
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02187123