Abstract
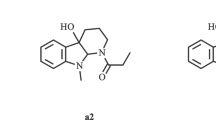
Analog compounds of the proposed intermediates of the biogenetic pathway to botrydial have been synthesized. These compounds were tested for their potential antifungal activity against the phytopathogenBotrytis cinerea. Our results showed a fungistatic effect of some compounds on mycelium growth. The most significant effect was exerted by 2-α-hydroxy-2,3-dihydro-1-epiprobotrydial, which inhibited growth ofB. cinerea. Some aspects of structure-activity relationships are discussed.
Similar content being viewed by others
References
Arpin, N., Favre-Bonvin, J., andThivend, S. 1977. Structure de la mycosporine 2, nouvelle molecule, isolée deBotrytis cinerea.Tetrahedron Lett. 10:819–820.
Bradshaw, A.P.W., andHanson, J.R. 1980. Three new sesquiterpenoid metabolites ofBotrytis cinerea.J. Chem. Soc., Perkin I 1980:741–743.
Coley-Smith, J.R., Verhoeff, K., andJarvis, W.R. 1980. The Biology of Botrytis. Academic Press, London. pp 42–63.
Collado, I.G., Aleu, J., Macias-Sánchez, A.J., andHernández-Galán, R. 1994. Inhibition ofBotrytis cinerea by new sesquiterpenoid compound from unusual rearrangement of isocaryophyllene.J. Nat. Prod. 57:738–746.
Cuevas, O., andHanson, J.R. 1977. Norbotrydial acetate, a nor-sesquiterpenoid aldehyde fromBotrytis cinerea.Phytochemistry 16:1061–1062.
Cutler, H.G., Springer, J.P., Arrendale, R.F., Arison, B.H., Cole, P.D., andRoberts, R.G. 1988. Cinereain: A novel metabolite with plant growth regulating properties fromBotrytis cinerea.Agric. Biol. Chem. 52:1725–1733.
Fehlhaber, H.-W., Geipel, R., Mercker, H.-S., Tschesche, R., andWelmar, K. 1974. Botrydial, ein Sesquiterpen-Antibiotikum aus der Nährlösung des Pilzes Botrytis cinerea.Chem. Ber. 107:1720–1730.
Gollnick, K., andSchade, G. 1970. The structure of an isocaryophyllene rearrangement product, 1,5,9,9-tetramethyl tricycle [6,2,1,04,11]undec-5-ene: X-ray analysis of the dibromo derivative.J. Chem. Soc., Chem. Commun. 1970:248–249.
Hanson, J.R. 1981. The biosynthesis of some sesquiterpenoids.Pure Appl. Chem. 53:1155–1162 (and references therein).
Kaiser, R., andLamparsky, D. 1976. Constituents of verbena oil. Part 2. Caryophyllene 2,6-β-oxide, a new sesquiterpenoid compound from the oil ofLippia citriodora Kunth.Helv. Chim. Acta 59:1803–1808.
Khomenko, T.M., Bagryanskaya, I.Y., Gatilov, Y.V., Korchagina, D.V., Gatilova, V.P., Dubovenko, Z.V., andBarkhash, V.A. 1985. Molecular rearrangements of isocaryophyllene in a superacid.Zh. Org. Khim. 21:677–678.
Kimata, T., Natsume, M., andMarumo, S. 1985. Botrydienal, a new phytotoxin and its related metabolites, dehydrobotrydienal and deacetyldihydrobotrydial produced byBotrytis cinerea.Tetrahedron Lett. 26:2097–2100.
Kimura, Y., Fujioka, H., Nakajima, H., Hamasaki, T., Irie, M., Fukuyama, K., andIsogai, A. 1986. Isolation, X-ray structure and biological activity of deacetyldihydrobotrydial produced byBotrytis squamosa.Agric. Biol. Chem. 50:2123–2135.
Lindner, H.J., andGross, B.v. 1977. Die Kristall-und Molekülstruktur von Dihydrobotrydial.Chem. Ber. 107:3332–3336.
Overeem, J.C., andVan Dijkman, A. 1968. Botrallin, a novel quinone produced byBotrytis alli.Recueil 87:940–944.
Patil, I.S., Kulkarni, S., andHegde, R.K. 1986. Bioassay of fungicides againstDrechlera Sorokiniana (Sacc).Pesticides 1986:30–31.
Suga, T., Hirata, T., Utsumi, R., andYosjioka, T. 1984. The metabolites ofBotrytis cinerea.J. Sci. Hiroshima Univ., Ser A 48:75–79.
Welmar, K., Tschesche, R., andBreitmaier, E. 1979. Botrylacton, ein neuer Wirkstoff aus der Nährlösung des PilzesBotrytis cinerea, 2.Chem. Ber. 112:3598–3602.
Whealer, B.E.J. 1969. An Introduction to Plant Diseases. John Wiley, London, pp. 187–188.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Collado, I.G., Aleu, J., Macías-Sánchez, A.J. et al. Synthesis and antifungal activity of analogues of naturally occurring botrydial precursors. J Chem Ecol 20, 2631–2644 (1994). https://doi.org/10.1007/BF02036197
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02036197