Abstract
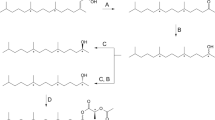
(R)- and (S)-10,14-dimethyl-1-pentadecyl isobutyrates were synthesized from (S)- and (R)-citronellols, respectively. TheR enantiomer was as active as the natural pheromone but theS enantiomer was less active in the electrophysiological analyses, which provided conclusive proof that the absolute configuration of the natural pheromone isR.
Similar content being viewed by others
References
Corey, E.J., Pasto, D.J., andMock, W.L. 1961. Chemistry of diimide. II. Stereochemistry of hydrogen transfer to carbon-carbon multiple bonds.J. Am. Chem. Soc. 83:2957–2958.
Dale, J.A., andMosher, H.S. 1973. Nuclear magnetic resonance enantiomer reagents. Configurational correlations via nuclear magnetic resonance chemical shifts of diastereomeric mandelate,O-methylmandelate, and α-methoxy-α-trifluoromethylphenylacetate (MTPA) esters.J. Am. Chem. Soc. 95:512–519.
Hoffman, J.M., Jr., andSchlessinger, R.H. 1971. Sodium metaperiodate: A mild oxidizing agent for the generation of di-imide from hydrazine.Chem. Commun. 1971:1245–1246.
Horiike, M., Tanouchi, M., andHirano, C. 1978. Synthesis of insect sex pheromones and their homologues: (Z)-6-alkenyl acetates from the Wittig reaction.Agric. Biol. Chem. 42:1963–1965.
Mori, K. 1994. Seirikasseitennenbutu no fuseigousei.Kagakutoseibutu 32:126–134 (in Japanese).
Mori, K., Harada, H., Zagatti, P., Cork, A., andHall, D.R. 1991. Pheromone synthesis, CXXVI. Synthesis and biological activity of four stereoisomers of 6,10,14-trimethyl-2-pentadecanol, the female-produced sex pheromone of rice moth (Corcyra cephalonica).Liebigs Ann. Chem. 1991:259–267.
Naoshima, Y., andMukaidani, H. 1987. Synthesis of racemate and enantiomers of 15-methyltri-triacontane, sex-stimulant pheromone of stable flyStomoxys calcitrans L.J. Chem. Ecol. 13:325–333.
Naoshima, Y., Munakata, Y, Yoshida, S., andFunai, A. 1991. Synthesis of chiral alcohol and ester pheromones through enzyme-catalysed hydrolysis usingPseudomonas fluorescens lipase: Preparation of (2R,6S,10S)-6,10,14-trimethylpentadecan-2-ol and the propionate of (2R,8R)-8-methyldecan-2-ol.J. Chem. Soc. Perkin Trans. 1:549–553.
Naoshima, Y., Munakata, Y., Funai, A., andYoshida, S. 1991. Synthesis of optically active natural products by lipase-catalyzed transformation, pp. 259–266, Symposium Papers of 33rd Symposium on the Chemistry of Natural Products.
Ogata, K. 1958. Studies on the Far Eastern urticating moth,Euproctis flava BREMER, as a pest of medical importance I. Morphological notes.Jpn. J. Sanit. Zool. 9:116–129.
Ohtani, I., Kusumi, T., Kashman, Y., andKakisawa, H. 1991. High field FT NMR application of Mosher's method. The absolute configurations of marine terpenoids.J. Am. Chem. Soc. 113:4092–4096.
Sruble, D.L., andArn, H. 1984. Combined gas chromatography and electroantennogram recording of insect olfactory responses, pp. 161–178,in H.E. Hummel and T.A. Miller (eds.).Techniques in Pheromone Research. Springer-Verlag, New York.
Wakamura, S., Yasuda, T., Ichikawa, A., Fukumoto, T., andMochizuki, F. 1994. Sex attractant pheromone of the tea Tussock moth,Euproctis pseudoconspersa (STRAND) (Lepidoptera: Lymantriidae): Identification and field attraction.Appl. Entomol. Zool. 29:403–411.
Wright, J., Drtina, G.J., Roberts, R.A., andPaquette, L.A. 1988. A convergent synthesis of triquinane sesterterpenes. Enantioselective synthesis of (-)-retigeranic acid A.J. Am. Chem. Soc. 110:5806–5817.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ichikawa, A., Yasuda, T. & Wakamura, S. Absolute configuration of sex pheromone for tea tussock moth,Euproctis pseudoconspersa (strand)via synthesis of (R)- and (S)-10, 14-dimethyl-1-pentadecyl isobutyrates. J Chem Ecol 21, 627–634 (1995). https://doi.org/10.1007/BF02033705
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02033705