Summary
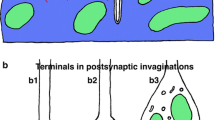
The frog neuromuscular junction was fixed and processed for electron microscopy according to the method of rapid freezing followed by freeze-substitution. The synaptic structures, including cleft material, paramembranous cytoplasmic coating on the postsynaptic membrane, and subsynaptic cytoplasmic elements, were examined in thin sections. The basal lamina, about 50 nm thick, was seen to bisect a synaptic cleft ∼100 nm wide. The lamina consists of two parts: the central dense line and the fine filaments protruding from it in the direction of the apposing postjunctional membrane. Present on the cytoplasmic surface of the postjunctional membrane are electron-dense protuberances, 41 ± 5 nm in width and 27 ± 5 nm in height (top to membrane centre). They are arranged in regular parallel rows, at 54 ± 4nm intervals (centre to centre). The paramembranous protuberance coats the inner surface of the postjunctional membrane at its apex as well as at the middle portion of the junctional process, pointing to its probable hairpin-like course in a transverse plane of the process. From its location and three-dimensional arrangement, this protuberance was termed the ‘postsynaptic arch’. A filamentous meshwork is present just beneath the postjunctional membrane and extends into the cell interior. The submembranous meshwork appears to connect to the underlying bundles of cytoskeletal filaments. The possibility is discussed that the postsynaptic, electron-dense arch corresponds to the 43-kDa protein, a major alkaline-extractable protein thought to be associated with the cholinergic receptor molecules in the postsynaptic membrane.
Similar content being viewed by others
References
ANDERSON-CEDERGREN, E. (1959) Ultrastructure of motor end plate and sarcoplasmic components of mouse skeletal muscle fibre as revealed by three dimensional reconstruction from serial sections.Journal of Ultrastructure Research, Suppl. 1, 1–191.
BIRKS. R., HUXLEY H. E. & KATZ, B. (1960) The fine structure of the neuromuscular junction of the frog.Journal of Physiology 150, 134–44.
CARTAUD, J. (1980) A critical re-evaluation of the structural organization of the excitable membrane inTorpedo marmorata electric organ. InOntogenesis and Functional Mechanisms of Peripheral Synapses (edited by TAXI, J.), pp. 199–210. Amsterdam, New York: Elsevier North-Holland.
CARTAUD, J., BENEDETTI, E. L., SOBEL, A. & CHANGEUX, J-P. (1978) A morphological study of the cholinergic receptor protein fromTorpedo marmorata in its membrane environment.Journal of Cell Science 29, 313–37.
CARTAUD, J. SOBEL, A., ROUSSELET, A., DEVAUX, P. E. & CHANGEUX, J-P. (1981) Consequences of alkaline treatment for the ultrastructure of the acetylcholine-receptor-rich membranes fromTorpedo marmorata electric organ.Journal of Cell Biology 90, 418–26.
COUTEAUX, R. (1960) Motor end-plate structure. InThe Structure and Function of Muscle, Vol. 1 (edited by BOURNE, G. M.), pp. 337–80. New York: Academic Press.
DREYER, F., PEPER, K., AKERT, K., SANDRI, C. & MOOR, H. (1973) Ultrastructure of the active zone in the frog neuromuscular junction.Brain Research 62, 373–80.
ELLIOT, J., BLANCHARD, S. G., WU, W., MOORE, H-P., RACS, J. & RAFFTERY, M. A. (1980) Purification ofTorpedo california post-synaptic membranes and fractionation of their constituent proteins.Biochemical Journal 185, 667–77.
ELLISMAN, M. H., RASH, J. A., STAEHELIN, L. A. & PORTER. K. R. (1976) Studies of excitable membranes. II. A comparison of specialization at neuromuscular junctions and non-junction sarcolemmas of mammalian fast and slow twitch muscle fibres.Journal of Cell Biology 68, 752–74.
FERTUCK, H. C. & SALPETER, M. M. (1976) Quantitation of junctional and extra-junctional acetylcholine receptors by electron microscopic autoradiography after125Iα-bungarotoxin binding at mouse neuromuscular junction.Journal of Cell Biology 69, 144–58.
FROEHNER, S. C., GULBRANDSEN, V., HYMAN, C., JENG, A. Y., NEUBIG, R. R. & COHEN, J. B. (1981) Immunofluorescence localization at the mammalian neuromuscular junction of the Mr 43,000 protein ofTorpedo postsynaptic membranes.Proceedings of the National Academy of Sciences USA 78, 5230–4.
GYSIN, R., WIRTH, M. & FLANAGAN, S. D. (1981) Structural heterogeneity and subcellular distribution of nicotinic synapse-associated proteins.Journal of Biological Chemistry 256, 11373–6.
HEUSER, J. E., REESE, T. S., DENNIS, M. J., JAN, L., YAN, Y. & EVANS, L. (1979) Synaptic exocytosis captured by quick-freezing and correlated with quantal transmitter release.Journal of Cell Biology 81, 275–300.
HEUSER, J. E. & SALPETER, S. R. (1979) Organization of acetylcholine receptors in quick-frozen, deep-etched, and rotary-replicatedTorpedo postsynaptic membrane.Journal of Cell Biology 82, 150–73.
HIROKAWA, N. & HEUSER, J. E. (1982) Internal and external differentiations of the postsynaptic membrane at the neuromuscular junction.Journal of Neurocytology 11, 487–510.
HIROKAWA, N. & KIRINO, T. (1980) An ultrastructural study of nerve and glial cells by freeze-substitution.Journal of Neurocytology 9, 243–54.
ROSENBLUTH, J. (1972) Myoneural junctions of two ultrastructurally distinct types in earthworm body wall muscle.Journal of Cell Biology 54, 566–79.
ROSENBLUTH, J. (1974) Substructure of amphibian motor endplate. Evidence for a granular component projecting from the outer surface of the receptive membrane.Journal of Cell Biology 62, 755–66.
SALPETER, M. M. & ELDEFRAWI, M. E. (1973) Sizes of end plate compartments, densities of acetylcholine receptor, and other quantitative aspects of neuromuscular transmission.Journal of Histochemistry and Cytochemistry 21, 769–78.
SEALOCK, R. (1980) Identification of regions of high acetylcholine receptor density in tannic acid-fixed postsynaptic membranes from electric tissue.Brain Research 199, 267–81.
SEALOCK, R. (1982) Visualization at the mouse neuromuscular junction of a submembrane structure in common withTorpedo postsynaptic membranes.Journal of Neuroscience 2, 918–23.
SEALOCK, R., WRAY, B. E. & FROEHNER, S. C. (1984) Ultrastructural localization of the Mr 43,000 protein and acetylcholine receptor inTorpedo postsynaptic membranes using monoclonal antibodies.Journal of Cell Biology 98, 2239–44.
SHOTTON, D. M., HEUSER, J. E., REESE, B. F. & REESE, T. S. (1979) Postsynaptic membrane folds of the frog neuromuscular junction visualized by scanning electron microscopy.Neuroscience 4, 427–35.
SOBEL, A., HEIDMAN, T., HOLFER, J. & CHANGEUX, J-P. (1978) Distinct protein components fromTorpedo marmorata membranes carry the acetylcholine receptor site and the binding site for local anesthetics and histrionicotoxin.Proceedings of the National Academy of Sciences USA 75, 510–14.
VAN HARREVELD, A. & CROWELL. J. (1964) Electron microscopy after rapid freezing on a metal surface and substitution fixation.Anatomical Record 149, 381–6.
VAN HARREVELD, A. & FIFKOVÁ, E. (1975) Rapid freezing of deep cerebral structures for electron microscopy.Anatomical Record 182, 377–86.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tatsuoka, H., Kadota, T. & Kono, K. Postsynaptic arch in the frog neuromuscular junction: Paramembranous protuberances coating the inner surface of the postjunctional membrane. J Neurocytol 17, 87–94 (1988). https://doi.org/10.1007/BF01735381
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01735381