Summary
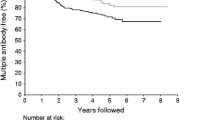
Insulin-dependent (Type I) diabetes mellitus is a chronic autoimmune disease. From studies in discordant twins and multiplex families a long prediabetic period has been reported. In a population-based program started in 1983, fifteen individuals at possible risk for future Type I diabetes were followed for up to 74 months. Two individuals (13%) developed Type I diabetes. These probands were characterized by the presence of high-level cytoplasmic islet cell antibodies (ICA), complement-fixing ICA, and an impaired first-phase insulin response after intravenous glucose load. Both were homozygous for a high-risk immunogenetic marker of Type I diabetes, i.e., non-Asp at codon 57 of the HLA-DQΒ chain. In all other subjects studied, the immunogenetic marker that confers “dominant resistance”, aspartic acid at codon 57, was found.
On the basis of our data we conclude that a combination of assays which determine ICA, first-phase insulin release, and HLA-DQB1 polymorphisms will identify individuals with a high probability of developing Type I diabetes at the population level. Conversely, HLA haplotypes positive for aspartic acid seem to confer resistance to the disease.
Similar content being viewed by others
Abbreviations
- HLA:
-
histocompatibility antigen
- IAA:
-
insulin autoantibodies
- ICA:
-
islet cell antibodies
- IVGTT:
-
intravenous glucose tolerance test
- SSC:
-
sodium saline citrate
References
Baisch JM, Weeks T, Giles R, Hoover M, Stasny P, Capra JD (1990) Analysis of HLA-DQ genotypes and susceptibility in insulin-dependent diabetes mellitus. N Engl J Med 322:1836–1841
Boehm BO, Scherbaum WA, Pfeiffer EF, Schöffling K (1987) Immungenetik des Typ I (insulinpflichtigen) Diabetes mellitus. Med Klin 82:439–442
Cavender DE, Wagener DK, Rabin BS (1984) The Pittsburgh insulin-dependent diabetes mellitus (IDDM) study. HLA antigens and haplotypes as risk factors for the development of IDDM in IDDM patients and their siblings. J Chron Dis 37:555–568
Chase HP, Voss MA, Butler-Simon N, Hoops S, O'Brien D, Doberson MJ (1987) Diagnosis of pre-type I diabetes. J Pediatr 111:807–812
Herskowitz RD, Wolfsdorf JI, Ricker AT, Vardi P, Dib S, Soeldner JS, Eisenbarth GS (1988) Transient hyperglycemia in childhood: identification of a subgroup with imminent diabetes. Diabetes Res 9:161–167
Lipton R, LaPorte RE (1989) Epidemiology of islet cell antibodies. Epidemiol Rev 11:182–203
Morel PA, Dorman JS, Todd JA, McDevitt HO, Trucco M (1988) Aspartic acid at position 57 of the HLA-DQΒ chain protects against type I diabetes: a family study. Proc Natl Acad Sci USA 85:8111–8115
Nepom GT (1990) HLA and type I diabetes. Immunol Today 11:314–315
Rosenbloom AL, Hunt SS (1982) Prognosis of impaired glucose tolerance in children with stress hyperglycemia, symptoms of hypoglycemia or asymptomatic glucosuria. J Pediatr 101:340–344
Rosenbloom AL, Hunt SS, Rosenbloom EK, MacLaren NK (1982) Ten-year prognosis of impaired glucose tolerance in siblings of patients with insulin-dependent diabetes. Diabetes 31:385–387
Saiki RK, Gelland DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, Mullis KB, Erlich HA (1988) Primer-directed enzymatic amplification of DNA with thermostable DNA polymerase. Science 239:487–491
Scherbaum WA, Mirakian R, Pujol-Borrell R, Dean BM, Bottazzo GF (1988) Immunocytochemistry in the study and diagnosis of organ-specific autoimmune disease. In: Polak JM, VanNoorden S (eds) Immunocytochemistry — modern methods and applications. Bristol, Wright, pp 456–476
Srikanta S, Ganda OP, Eisenbarth GS, Soeldner JS (1983) Islet cell antibodies and beta-cell function in monozygotic triplets and twins discordant for type I diabetes mellitus. New Engl J Med 322:322–325
Tarn AC, Thomas JM, Dean BM, Ingram D, Schwarz G, Bottazzo GF, Gale EAM (1988) Predicting insulin-dependent diabetes. Lancet i:845–850
Todd JA, Bell JI, McDevitt HO (1987) HLA-DQΒ gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature 329:599–604
Trucco M, Dorman JS (1989) Immunogenetics of insul-independent diabetes mellitus in humans. CRC Critical Rev Immunol 9:201–245
VanRood JJ, Van Leeuwen A, Ploem JS (1976) Simultaneous detection of two cell populations by two color fluorescence and application to the recognition of B-cell determinants. Nature 262:795–797
Ziegler AG, Herskowitz RD, Jackson RA, Soeldner JS, Eisenbarth GS (1990) Predicting type I diabetes. Diabetes Care 13:762–775
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Boehm, B.O., Manfras, B., Rosak, C. et al. Aspartic acid at position 57 of the HLA-DQβ chain is protective against future development of insulin-dependent (Type 1) diabetes mellitus. Klin Wochenschr 69, 146–150 (1991). https://doi.org/10.1007/BF01665854
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01665854