Abstract
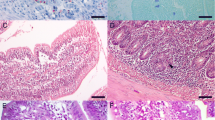
We examined mucosal injury in the jejunum of the rat during infection with the nematode parasite, Nippostrongylus brasiliensis (Nb). Injury was documented morphologically (increase in crypt length with or without villus atrophy) and biochemically (activities of digestive or proliferative enzymes) and related to mast cell activation and leukotriene generation. At day 4 crypt length and thymidine kinase activity were increased; no changes in villus parameters were recorded. No evidence of mast cell activation was found and leukotriene levels in the mucosa were normal. At day 7, the gut was acutely inflamed and edema was present at the tips of the villi. This progressed to enterocyte detachment, resulting in villus atrophy with decreased activities of brush border enzymes. At this stage mucosal histamine was decreased and rat mast cell protease II (RMCP II) was increased in serum, indicating mast cell activation. In addition, mucosal leukotrienes (LTB 4,LTC 4,LTE 4 ) were present in significant quantities. Following worm expulsion, the villus abnormalities resolved and serum RMCP II returned to normal. However, the crypt hyperplasia persisted. Our results suggest that during Nb infection at least two components of injury can be identified. One component, epithelial injury at the villus tips, may be related to activation of mucosal mast cells.
Similar content being viewed by others
References
May RJ, Quaroni A, Kirsch K, Isselbacher K: A villous cell-derived inhibitor of intestinal cell proliferation. Am J Physiol 241:G520-G527, 1981
Kerzner B, Kelly MH, Butler DG, Gall DG, Hamilton JR: Transmissible gastroenteritis: Sodium transport and the intestinal epithelium during the course of viral enteritis. Gastroenterology 72:457–461, 1977
Ferguson A, Jarrett EEE: Hypersensitivity reactions in the small intestine. I. Thymus dependence of experimental “partial villous atrophy”. Gut 16:114–117, 1975
Guy-Grand D, Vassalli P: Gut injury in mouse graft-versus-host reaction. Study of its occurrence and mechanisms. J Clin Invest 77:1584–1595, 1986
MacDonald TT, Spencer JM: Evidence that activated mucosal T cells play a role in the pathogenesis of enteropathy in human small intestine. J Exp Med 167:1341–1349, 1988
Bienenstock J, Befus AD, Denburg JA: Mast cell heterogeneity: Basic questions and clinical implications.In Mast Cell Differentiation and Heterogeneity. AD Befus, J Bienenstock, JA Denburg (eds). New York, Raven Press, 1986, pp 391–402
King SJ, Miller HRP: Anaphylactic release of mucosal mast cell protease and its relationship to gut permeability inNippostrongylus-primed rats. Immunology 51:653–660, 1984
Russell DA, Castro GA: Physiology of the gastrointestinal tract in the parasitized host,In Physiology of the Gastrointestinal Tract, 2nd ed. L Johnson (ed). New York, Raven Press, 1987, pp 1749–1780
Ramage JK, Hunt RH, Perdue MH: Changes in intestinal permeability and epithelial differentiation during inflammation in the rat. Gut 29:57–61, 1988
Ramage JK, Stanisz A, Scicchitano R, Hunt RH, Perdue MH: Effect of immunological reactions on rat intestinal epithelium. Correlation of increased gut permeability to51Cr-EDTA and ovalbumin during inflammation and anaphylaxis in the rat. Gastroenterology 94:1368–1375, 1988
Woodbury RG, Miller HRP, Huntley JF, Newlands GFJ, Palliser AC, Wakelin D: Mucosal mast cells are functionally active during expulsion of intestinal nematode infections in rat. Nature 312:450–452, 1984
Heavey DJ, Ernst PB, Stevens RL, Befus AD, Bienenstock J, Austen KF: Generation of leukotriene C4, leukotriene B4, and prostaglandin D2 by immunologically activated rat intestinal mucosal mast cells. J Immunol 140:1953–1957, 1988
Nawa Y, Miller HRP: Protection againstNippostrongylus brasiliensis by adoptive immunization with immune thoracic duct lymphocytes. Cell Immunol 37:51–60, 1978
Perdue MH, Chung M, Gall DG: The effect of intestinal anaphylaxis on gut function in the rat. Gastroenterology 86:391–397, 1984
Muller M, Sorrell TC: Quantification of sulfidopeptide leukotrienes by reverse-phase high-performance liquid chromatography. J Chromatogr 343:213–218, 1985
Miller HRP, Woodbury RG, Huntley JK, Newlands G: Systemic release of mucosal mast cell protease in primed rats challenged withNippostrongylus brasiliensis. Immunology 49:471–479, 1983
MacQueen G, Marshall J, Perdue M, Seigal S, Bienenstock J: Conditioned secretion of rat mast cell protease II by mucosal mast cells. Science 243:83–84, 1989
Weiser MM: Intestinal epithelial cell surface glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem 248:2536–2541, 1973
Befus AD, Johnson N, Bienenstock J:Nippostrongylus brasiliensis: Mast cells and histamine levels in tissues of infected and normal rats. Exp Parasitol 48:1–8, 1979
Smith MW: Expression of digestive and absorptive function in differentiating enterocytes. Annu Rev Physiol 47:247–260, 1985
Symons LEA: Kinetics of the epithelial cells, and the morphology of villi and crypts in the jejunum of the rat infected by the nematodeNippostrongylus brasiliensis. Gastroenterology 49:158–168, 1965
Symons LEA, Fairbairn D: Pathology, absorption, transport and activity of digestive enzymes in rat jejunum parasitized by the nematodeNippostrongylus brasiliensis. Fed Proc 21:913–918, 1962
Urquhart GM, Mulligan W, Eadie RM, Jennings FW: Immunologic studies on Nippostrongylus brasiliensis infection in the rat: The role of local anaphylaxis. Exp Parasitol 17:210–217, 1964
Perdue MH, Forstner JF, Roomi N, Gall DG: Epithelial response to intestinal anaphylaxis in rats: goblet cell secretion and enterocyte damage. Am J Physiol 247:G632-G637, 1984
Lake AM, Kagey-Sobotka A, Jakubowicz T, Lichtenstein LM: Histamine release in acute anaphylactic enteropathy of the rat. J Immunol 133:1529–1534, 1984
Freier S, Eran M, Goldstein R: The effect of immediate type gastrointestinal allergic reactions on brush border enzymes and gut morphology in the rat. Pediatr Res 19:456–459, 1985
Sjolander A, Magnusson KE: Effect of antigen challenge on intestinal permeability and morphology in rats immunized with gliadin or ovalbumin. Int Arch Allergy Appl Immunol 84:284–290, 1987
Sage H, Woodbury RG, Bornstein P: Structural studies of human type IV collagen. J Biol Chem 254:9893–9900, 1979
Patrick MK, Dunn IJ, Buret A, Miller HRP, Huntley JF, Gibson S, Gall DG: Mast cell protease release and mucosal ultrastructure during intestinal anaphylaxis in the rat. Gastroenterology 94:1–9, 1988
Murray M, Jarrett WFH, Jennings FW: Mast cells and macromolecular leak in intestinal immunological reactions: The influence of sex of rats infected withNippostrongylus brasiliensis. Immunology 21:17–31, 1971
Cobden I, Rothwell J, Axon ATR: Intestinal permeability in rats infected withNippostrongylus brasiliensis. Gut 20:716–721, 1979
Bjarnason I, Smethurst P, Levi AJ, Peters TJ: Intestinal permeability to51Cr-EDTA in rats with experimentally induced enteropathy. Gut 26:579–585, 1985
Schwartz LB, Austen KF: Structure and function of the chemical mediators of mast cells. Prog Allergy 34:271–321, 1984
Moqbel R, King SJ, MacDonald AJ, Miller HRP, Cromwell O, Shaw RJ, Kay AB: Enteral and systemic release of leukotrienes during anaphylaxis ofNippostrongylus brasiliensis-primed rats. J Immunol 137:296–301, 1986
Weller PF, Lee CW, Foster DW, Corey EJ, Austen KF, Lewis RA: Generation and metabolism of 5-lipoxygenase pathway leukotrienes by human eosinophils: Predominant production of leukotriene C4. Proc Natl Acad Sci USA 80:7626–7630, 1984
Manson-Smith DF, Bruce RG, Parrott DMV: Villous atrophy and expulsion of intestinalTrichinella spiralis are mediated by T cells. Cell Immunol 47:285–292, 1979
Hanglow AC, Perdue MH, Dyck N, Bienenstock JB: Role of non-specific cytotoxic cells in intestinal epithelial cell injury inNippostrongylus brasiliensis infection. Clin Immunol Immunopathol 46:406–411, 1988
Author information
Authors and Affiliations
Additional information
This research was supported by grants from the Medical Research Council of Canada and the Canadian Foundation for Ileitis and Colitis.
Rights and permissions
About this article
Cite this article
Perdue, M.H., Ramage, J.K., Burget, D. et al. Intestinal mucosal injury is associated with mast cell activation and leukotriene generation duringNippostrongylus-induced inflammation in the rat. Digest Dis Sci 34, 724–731 (1989). https://doi.org/10.1007/BF01540344
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01540344