Summary
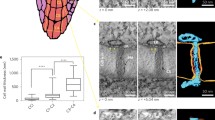
In this report we show that large cytoplasmic channels form between the tapetal cells ofZea mays (maize) during the period of tapetal cell differentiation. Tapetal cells are connected by plasmodesmata through their cellulosic cell walls prior to the first meiotic division of the meiocytes. As the tapetal cellulose wall is degraded at the onset of meiosis, both plasmodesmata and cytoplasmic channels measuring 50–200 nm are detectable between tapetal cells. By the time the meiotic tetrad is formed, the cytoplasmic channels are well-established and vary in size from 100–400 nm. The channels, with an average diameter of 200–300 nm, persist after the microspores are released from the callose wall and throughout the period of exine development in microsporogenesis. The channels could potentially allow for free exchange of cytoplasm and organelles. As the tapetal cells begin to pull apart and become vacuolate prior to microspore mitosis, the connecting channels are no longer detectable.
Similar content being viewed by others
References
Behnke H-D (1969) Über den Feinbau und die Ausbreitung der Siebröhren-Plasmafilamente und über Bau und Differenzierung der Siebporen bei einigen Monocotylen und beiNuphar. Protoplasma 68: 377–402
Chapman GP (1987) The tapetum. Int Rev Cytol 107: 111–125
D'Amato F (1984) Role of polyploidy in reproductive organs and tissues. In: Johri BM (ed) Embryology of angiosperms. Springer, Berlin Heidelberg New York Tokyo, pp 519–566
Dickinson HG, Bell PR (1976) The changes in the tapetum ofPinus banksiana accompanying formation and maturation of the pollen. Ann Bot 40: 1101–1109
Eleftheriou EP (1990) Monocotyledons. In: Behnke H-D, Sjolund RD (eds) Sieve elements: comparative structure, induction and development. Springer, Berlin Heidelberg New York Tokyo, pp 139–159
Gunning BES, Overall RL (1983) Plasmodesmata and cell-to-cell transport in plants. Bioscience 33: 260–265
Heslop-Harrison J (1964) Cell walls, cell membranes and protoplasmic connections during meiosis and pollen development. In: Linskens HF (ed) Pollen physiology and fertilization. North-Holland, Amsterdam, pp 39–47
— (1966) Cytoplasmic continuities during spore formation in flowering plants. Endeavor 25: 65–72
— (1972) Sexuality of angiosperms. In: Steward FC (ed) Plant physiology. Academic Press, London, pp 133–289
Mariani C, De Beuckeleer M, Treuttner J, Leemans J, Goldberg RB (1990) Induction of male sterility in plants by a chimeric ribonuclease gene. Nature 347: 737–741
Moss GI, Heslop-Harrison J (1967) A cytochemical study of DNA, RNA and protein in the developing maize anther. Ann Bot 31: 555–575
Murgia M, Charzynska M, Rougier M, Cresti M (1991) Secretory tapetum ofBrassica oleracea L.: polarity and ultrastructural features. Sex Plant Reprod 4: 28–35
Pacini EG (1990) Tapetum and microspore function. In: Blackmore S, Knox RB (eds) Microspores evolution and ontogeny. Academic Press, London, pp 213–237
—, Franchi G, Hesse M (1985) The tapetum: its form, function, and possible phytogeny in Embryophyta. Plant Syst Evol 149: 155–185
Parthasarathy MV (1974) Ultrastructure of phloem in palms. Protoplasma 79: 93–125
Steiglitz H (1977) Role of β-1.3-glucanase in postmeiotic microspore release. Dev Biol 57: 87–97
Worrall D, Hird DL, Hodge R, Paul W, Draper J, Scott R (1992) Premature dissolution of the microsporocyte callose wall causes male sterility in transgenic tobacco. Plant Cell 4: 759–771
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Perdue, T.D., Loukides, C.A. & Bedinger, P.A. The formation of cytoplasmic channels between tapetal cells inZea mays . Protoplasma 171, 75–79 (1992). https://doi.org/10.1007/BF01379282
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01379282