Summary
The results of this work clarify several structural and temporal aspects of biogenesis of the basal body-root complex inChlamydomonas reinhardtii. The two phases of basal body development (probasal body assembly and conversion of probasal body into mature basal body) occur at identical mitotic stages in successive mitoses during multiple fission, which indicates a tight coupling between basal body development and the mitotic cycle. The two steps of basal body development are separated from one another in time,i.e. immature probasal bodies originate during an interval lasting ca. 5 min between mid-metaphase and early telophase, but mature after a quasi-dormant period only during early prophase of the next mitotic round. The duration of the dormant period depends on the interval between two mitoses: during synchronized vegetative growth there is an interval of ca. 20 h (interphase growth) between two rounds of multiple fissions, but only a maximum interval of 1.5 h between the successive mitoses of one round of multiple fissions.
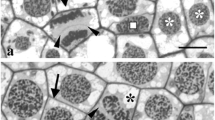
The microtubular root system, which is bisected at the same time as the basal body apparatus in a plane perpendicular to the distal connecting fiber during prophase, and whose roots seem to be reduced in length, starts duplication at early metaphase with the successive origin of two short bud-like partner roots just opposite the remnants. These initial roots elongate during subsequent phases by unilateral and radial growth from the basal bodies and along the cell's periphery, but exactly where they terminate is not known. The two-stranded roots opposite each other appear to be again connected as early as anaphase.
The striation pattern of the distal connecting fiber is lost during early prophase thus indicating a partial breakdown of the fiber.
Similar content being viewed by others
References
Adams GMW, Wright RL, Jarvik JW (1985) Defective temporal and spatial control of flagellar assembly in a mutant ofChlamydomonas reinhardii with variable flagellar number. J Cell Biol 100: 955–964
Aitchison WA, Brown DL (1986) Duplication of the flagellar apparatus and cytoskeletal microtubule system in the algaPolytomella. Cell Motility Cytoskel 6: 122–127
Belar K (1926) Der Formwechsel der Protistenkerne. Ergeb Fortschritte Zool 6: 235–654
Cavalier-Smith T (1974) Basal body and flagellar development during the vegetative cell cycle and the sexual cycle ofChlamydomonas reinhardii. J Cell Sci 16: 529–556
Coss RA (1974) Mitosis inChlamydomonas reinhardii basal bodies and the mitotic apparatus. J Cell Biol 63: 325–329
Craigie RA, Cavalier-Smith T (1982) Cell volume and the control of theChlamydomonas cell cycle. J Cell Sci 54: 173–191
Gaffal KP (1987) Mitosis-specific oscillations of mitochondrial morphology inChlamydomonas reinhardii. Endocyt Cell Res 4: 41–62
—,Schneider GJ (1980) Morphogenesis of the plastidome and the flagellar apparatus during the vegetative life cycle of the colourless phytoflagellatePolytoma papillatum. Cytobios 27: 43–61
Goodenough UW, Weiss RL (1978) Interrelationships between microtubules, a striated fiber, and gametic mating structure ofChlamydomonas reinhardii. J Cell Biol 76: 430–438
Gould RR (1975) The basal bodies ofChlamydomonas reinhardii. Formation from probasal bodies, isolation, and partial characterization. J Cell Biol 65: 65–74
Gruber HE (1978) X-ray and proton-induced ultrastructural changes in dividingChlamydomonas reinhardi. Radiat Res 73: 137–159
Hoops HJ, Witman GB (1983) Outer doublet heterogeneity reveals structural polarity related to beat direction inChlamydomonas flagella. J Cell Biol 97: 902–908
—,Wright RL, Jarvik JW, Witman GB (1984) Flagellar waveform and rotational orientation in aChlamydomonas mutant lacking normal striated fibers. J Cell Biol 98: 818–824
Huang B, Ramanis Z, Dutcher SK, Luck DJL (1982) Uniflagellar mutants ofChlamydomonas: evidence for the role of basal bodies in transmission of positional information. Cell 29: 745–753
Johnson U, Porter KR (1968) Fine structure of cell division inChlamydomonas reinhardi. Basal bodies and microtubules. J Cell Biol 38: 403–425
Kamiya R, Witman GB (1984) Submicromolar levels of calcium control the balance of beating between the two flagella in demembranated models ofChlamydomonas. J Cell Biol 98: 97–107
Kates JR, Chiang KS, Jones RT (1968) Studies on DNA replication during synchronized vegetative growth and gametic differentiation inChlamydomonas reinhardtii. Exp Cell Res 49: 121–135
Marano F (1976) Étude ultrastructurale de la division chezDunaliella. J Microsc Biol Cell 25: 279–282
—,Santamaria H, Fries W (1984) Effects of diazepam on mitosis and basal body duplication of synchronously dividing flagellate cells. Biol Cell 50: 163–172
Melkonian M (1984) Flagellar apparatus ultrastructure in relation to green algal classification. In:Irvine DEG, John DM (eds) Systematics of the Green Algae. Academic Press, London, Syst Assoc Spec Vol 27: 73–120
—,Reize IB, Preisig HR (1987) Maturation of a flagellum/basal body requires more than one cell cycle in algal flagellates: studies onNephroselmis olivacea (Prasinophyceae). In:Wiessner W, Robinson DG, Starr RC (eds) Molecular and cellular aspects of algal development. Springer, Berlin Heidelberg, pp 102–113
—,Robenek H (1984) The eyespot apparatus of flagellated green algae: a critical review. Progr Phycol Res 3: 193–268
Ringo DL (1967) Flagellar motion and fine structure of the flagellar apparatus inChlamydomonas. J Cell Biol 33: 543–571
Surzycki S (1971) Synchronously grown cultures ofChlamydomonas reinhardi. In:San Pietro A (ed) Methods in enzymology, Vol 23. Academic Press, New York, pp 67–73
Weiss RL (1984) Ultrastructure of the flagellar roots inChlamydomonas gametes. J Cell Sci 67: 133–143
Wolf KW (1984) Qualitative and quantitative Analyse ultradünner Serienschnitte durch verschiedene Entwicklungsstadien aus dem vegetativen und generativen Zellzyklus des heterotrophen PhytoflagellatenPolytoma papillatum unter besonderer Berücksichtigung des Mikrotubuliinventars. Dissertation, Universität Erlangen — Nürnberg
Wright RL, Chojnacki B, Jarvik JW (1983) Abnormal basal-body number, location, and orientation in a striated fiber-defective mutant ofChlamydomonas reinhardtii. J Cell Biol 96: 1697–1707
Author information
Authors and Affiliations
Additional information
Dedicated to Prof. Dr. C.-G. Arnold (Erlangen) on the occasion of his 60th birthday.
Rights and permissions
About this article
Cite this article
Gaffal, K.P. The basal body-root complex ofChlamydomonas reinhardtii during mitosis. Protoplasma 143, 118–129 (1988). https://doi.org/10.1007/BF01291156
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01291156