Abstract
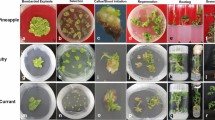
A procedure for the production of fertile transgenic brassicas via Ri-mediated transformation is reported in this paper. Transgenic hairy root lines were selected for 12 vegetable brassica cultivars and lines representing six varieties: broccoli, Brussels sprouts, cabbage, cauliflower, rapid-cycling (allBrassica oleracea) and Chinese cabbage (B. campestris). Leaf explants or petioles of intact cotyledons were co-cultivated withAgrobacterium strain A4T harbouring various binary vectors. The T-DNA region of all binary vectors contained a neomycin phosphotransferase II gene for kanamycin resistance, in addition to other genes. Hairy root lines grew prolifically on hormone-free medium containing kanamycin. Transgenic shoots were regenerated from all cultivars either spontaneously or after transfer of hairy roots to a hormone-containing medium. Southern analysis confirmed that the plants were transgenic. Plants from all brassica types were successfully transferred to greenhouse conditions. Plants were fertile and segregation analysis confirmed transmission of traits to progeny.
Similar content being viewed by others
Abbreviations
- BA :
-
6-Benzylaminopurine
- GUS :
-
β-Glucuronidase
- LS :
-
Linsmaier and Skoog medium
- NAA :
-
I-Naphthaleneacetic acid
- NPTII :
-
Neomycin phosphotransferase II
- TDZ :
-
thidiazuron
References
An G, Ebert PR, Mitra A, Ha SB (1988) Binary vectors. In: Gelvin SB, Schilperoort RA (eds) Plant molecular biology manual. Kluwer, Dordrecht, pp A3/1–19
Berthomieu P, Jouanin L (1992) Transformation of rapid cycling cabbage (Brassica oleracea var.capitata) withAgrobacterium rhizogenes. Plant Cell Rep 11:334–338
Boulter ME, Croy E, Simpson P, Shields R, Croy RRD, Shirsat AH (1990) Transformation ofBrassica napus L. (oilseed rape) usingAgrobacterium tumefaciens andAgrobacterium rhizogenes — a comparison. Plant Sci 70:91–99
Burow MD, Chlan CA, Sen P, Lisca A, Murai N (1990) High-frequency generation of transgenic tobacco plants after modified leaf disc cocultivation withAgrobacterium tumefaciens. Plant Mol Biol Rep 8:124–139
Christey MC (1997) Transgenic crop plants usingAgrobacterium rhizogenes-mediated transformation. In: Doran PM (ed) Hairy roots: culture and applications. Harwood, Amsterdam, pp 99–111
Christey MC, Sinclair BK (1992) Regeneration of transgenic kale (Brassica oleracea var.acephala), rape (B. napus) and turnip (B. campestris var.rapifera) plants viaAgrobacterium rhizogenes mediated transformation. Plant Sci 87:161–169
Damgaard O, Rasmussen O (1991) Direct regeneration of transformed shoots inBrassica napus from hypocotyl infections withAgrobacterium rhizogenes. Plant Mol Biol 17:1–8
David C, Tempe J (1988) Genetic transformation of cauliflower (Brassica oleracea L. var.botrytis) byAgrobacterium rhizogenes. Plant Cell Rep 7:88–91
Gleave AP (1992) A versatile binary vector system with a T-DNA organisational structure conducive to efficient integration of cloned DNA into the plant genome. Plant Mol Biol 20:1203–1207
Grant JE, Dommisse EM, Christey MC, Conner AJ (1991) Gene transfer to plants usingAgrobacterium. In: Murray DR (ed) Advanced methods in plant breeding and biotechnology. CAB International, Wallingford, pp 50–73
Guerche P, Jouanin L, Tepfer D, Pelletier G (1987) Genetic transformation of oilseed rape (Brassica napus) by the Ri T-DNA ofAgrobacterium rhizogenes and analysis of inheritance of the transformed phenotype. Mol Gen Genet 206:382–386
Hatamoto H, Boulter ME, Shirsat AH, Croy EJ, Ellis JR (1990) Recovery of morphologically normal transgenic tobacco from hairy roots co-transformed withAgrobacterium rhizogenes and a binary vector plasmid. Plant Cell Rep 9:88–92
Jun SI, Kwon SY, Paek KY, Paek KH (1995)Agrobacterium-mediated transformation and regeneration of fertile transgenic plants of chinese cabbage (Brassica campestris spp.pekinensis cv. “Spring Flavor”). Plant Cell Rep 14:620–625
Lassner MW, Peterson P, Yoder JI (1989) Simultaneous amplification of multiple DNA fragments by polymerase chain reaction in the analysis of transgenic plants and their progeny. Plant Mol Biol Rep 7:116–128
Linsmaier EM, Skoog F (1965) Organic growth factor requirements of tobacco tissue cultures. Physiol Plant 18:100–127
Montanelli C, Nascari G (1991) Introduction of an antibacterial gene in potato (Solanum tuberosum L.) using a binary vector inAgrobacterium rhizogenes. J Genet Breed 45:307–316
Moore L, Warren G, Strobel G (1979) Involvement of a plasmid in the hairy root disease of plants caused byAgrobacterium rhizogenes. Plasmid 2:617–626
Noda T, Tanaka N, Mano Y, Nabeshima S, Ohkawa H, Matsui C (1987) Regeneration of horseradish hairy roots incited byAgrobacterium rhizogenes infection. Plant Cell Rep 6:283–286
Tepfer D (1989) Ri T-DNA fromAgrobacterium rhizogenes: a source of genes having applications in rhizosphere biology and plant development, ecology, and evolution. In: Kosuge T, Nester EW (eds) Plant-microbe interactions. Molecular and genetic perspectives, vol 3, McGraw-Hill, New York, pp 294–342
Timmerman GM, Frew TJ, Miller AL, Weedon NF, Jermyn WA (1993) Linkage mapping ofsbm-1, a gene conferring resistance to pea seed-borne mosaic virus, using molecular markers inPisum sativum. Theor Appl Genet 85:609–615
Webb KJ, Jones S, Robbins MP, Minchin FR (1990) Characterization of transgenic root cultures ofTrifolium repens, Trifolium pratense andLotus corniculatus and transgenic plants ofLotus corniculatus. Plant Sci 70:243–254
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Phillips
Rights and permissions
About this article
Cite this article
Christey, M.C., Sinclair, B.K., Braun, R.H. et al. Regeneration of transgenic vegetable brassicas (Brassica oleracea andB. campestris) via Ri-mediated transformation. Plant Cell Reports 16, 587–593 (1997). https://doi.org/10.1007/BF01275497
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01275497