Summary
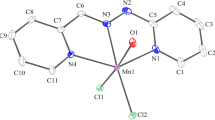
The complexes of MnII, CoII, NiII, CuII, ZnII, CdII, HgII, CoIII and UO 2+2 ions with 2-hydroxyimino-3-(2′-hydrazonopyridyl)-butane (HL) have been synthesised and characterized by elemental analyses, molar conductivities, magnetic measurements and spectral (i.r., visible, n.m.r.) studies. I.r. spectra show that HL behaves as a neutral or mononegative ligand and binds in a bidentate and/or tridentate manner. Also, HL behaves as oxidizing agent towards CoII forming diamagnetic CoIII complexes depending on the preparative conditions. Different stereochemistries are proposed for MnII, CoIII, CoII, NiII and CuII on the basis of spectral and magnetic studies.
Similar content being viewed by others
References
K. M. Ibrahim, A. A. El-Asmy, M. M. Bekheit and M. M. Mostafa,Transition Met. Chem.,10, 175 (1985).
G. M. Abu-El-Reash, K. M. Ibrahim and M. M. Bekheit,Transition Met. Chem.,15, 148 (1990).
K. M. Ibrahim, A. A. El-Asmy, M. M. Bekheit and M. M. Mostafa,Acta Chim. Hung.,119, 355 (1985).
K. M. Ibrahim, M. M. Bekheit, G. M. Abu-El-Reash and M. M. Mostafa,Polyhedron,5, 1635 (1986).
W. J. Geary,Coord. Chem. Rev.,7, 81 (1971).
B. F. Little and G. J. Long,Inorg. Chem.,17, 3401 (1978).
S. Burman and D. N. Sathyanaryana,J. Coord. Chem.,11, 219 (1982).
G. C. Percy and D. A. Thornton,Inorg. Nucl. Chem. Lett.,7, 599 (1971).
M. Mohan and B. D. Paramhans,Indian J. Chem.,19A, 759 (1980).
M. Mohan and M. Kumar,Transition Met. Chem.,10, 255 (1985).
L. V. Sudha and N. D. Sathyanaryana,J. Coord. Chem.,13, 207 (1984).
K. Nakamoto,Infrared Spectra of Inorganic and Coordination Compounds, Wiley, New York, 1970.
A. T. T. Hsich, R. M. Sheahan and B. O. West,Aust. J. Chem.,28, 885 (1975).
S. P. McGlynn, J. K. Smith and W. C. Neely,J. Chem. Phys.,35, 105 (1961).
A. N. Speca, N. M. Karayanis and L. L. Pytlewski,Inorg. Chem. Acta,9, 87 (1974).
B. Beecroft, M. J. M. Campbell and R. Grzeskowia,J. Inorg. Nucl. Chem.,36, 55 (1974).
J. R. Ferraro,Low Frequency Vibrations of Inorganic and Coordination Compounds, Plenum Press, New York, 1971.
H. Morita, Y. Nakamura and S. Kawaguchi,Bull. Chem. Soc. Japan,45, 2468 (1972).
C. K. Jørgensen,Acta Chim. Scand.,10, 887 (1956).
A. B. P. Lever,Inorganic Electronic Spectroscopy, Elsevier, Amsterdam, 1968.
K. M. Ibrahim and M. M. Bekheit,Transition Met. Chem.,13, 230 (1988).
P. K. Panda, S. B. Mishra and B. K. Mohapatka,J. Inorg. Nucl. Chem.,42, 497 (1980).
D. H. L. Goodgame and F. A. Cotton,J. Chem. Soc., 3735 (1961).
M. Palaniandavar and C. Natarajan,Aust. J. Chem.,33, 737 (1980).
S. P. McGlynn and J. K. Smith,J. Molec. Spectorc.,6, 164 (1961).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ibrahim, K.M., Bekheit, M.M. & El-Reash, G.M.A. Transition metal complexes derived from 2-hydroxyimino-3-(2′-hydrazonopyridyl)-butane. Transition Met Chem 16, 189–192 (1991). https://doi.org/10.1007/BF01032831
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01032831