Abstract
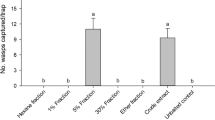
Male moths belonging to 17 species of Geometridae and nine species of Noctuidae were captured in traps baited with synthetic chemicals as part of a field screening program. The compounds tested were the C18-C22 homologs of: (1) (3Z,6Z,9Z)-triene hydrocarbons; (2) mixtures containing equal quantities of (3Z,6Z)-cis-9,10-expoxydienes, (3Z,92Z)-cis-6,7-epoxydienes, and (6Z,9Z)-cis-3,4-epoxydienes; (3) (3Z,6Z)-9S,10R-epoxydienes; (4) (3Z,6Z)-9R,10S-epoxydienes; and (5) (3Z,6Z,9Z,11E)-nonadecatetraene. Field captures and electroantennographic assays revealed a high degree of specificity in the responses of many species to the synthetic chemicals. In several species the ability of males to discriminate between the 9S,10R and 9R,10S enantiomers of the monoepoxydiene isomers was clearly shown. Synergists and inhibitors were discovered for several of the reported attractants, some of which were not previously known to have semiochemical activity. The geometrid moths captured includedEpirrhoe sperryi (Herbulot),Mesoleuca ruficillata (Guenée),Triphosa haesitata (Guenée),Metanema inatomaria (Guenée),Prochoerodes transversata (Drury),Cabera erythemaria (Guenée),Synaxis jubararia (Hulst),Dysstroma brunneata ethela (Hulst),Eulithes testata (Linnaeus),Sicya macularia (Harris),Xanthorhoe iduata (Guenée),X. abrasaria aquilonaria (Herrich-Schäffer),X. munitata (Hübner),Itame loricaria (Eversmann),Eupithecia annulata (Hulst),E. rovocastaliata (Packard) andE. satyrata dodata (Taylor). The noctuid moths captured includedBleptina caradrinalis (Guenée),Idia américalis (Guenée),I. aemula (Hübner),Rivula propinqualis (Guenée),Lomanaltes eductalis (Walker),Spargaloma sexpunctata (Grote),Caenurgina distincta (Neumuller),Euclidia cuspidea (Hübner), andZale duplicata (Bethune). Six of the nine noctuid species captured belong to three subfamilies for which sex attractants had not been reported previously. Details for the stereospecific synthesis of (3Z,6Z)-cis-9,10-epoxydienes are also reported.
Similar content being viewed by others
References
Becker, D., Kimmel, T., Cyjon, R., Moore, I., Wysoki, M., Bestmann, H.J., Platz, H., Roth, K., andVostrowsky, O. 1983. (3Z,6Z,9Z)-3,6,9-Nonadecatriene—a component of the sex pheromonal system of the giant looper,Boarmia (Ascotis)selenaria Schiffermüller (Lepidoptera: Geometridae).Tetrahedron Lett. 24:5505–5508.
Bestmann, H.J., Brosche, T., Koschatzky, K.H., Michaelis, K., Platz, H., Roth, K., Suss, J., Vostrowsky, O., andKnauf, W. 1982. 1,3,6,9-Nonadecatetraen, das Sexualpheromon des FrostspannersOperophtera brumata (Geometridae).Tetrahedron Lett. 23:4007–4010.
Bierl, B.A., Beroza, M., andCollier, W. 1970. Potent sex attractant of the gypsy moth: Its isolation, identification, and synthesis.Science 170:87–89.
Brand, J.M., Young, J. Chr., andSilverstein, R.M. 1979. Insect pheromones: A critical review of recent advances in their chemistry, biology, and application, pp. 1–189,in W. Herz, H. Grisebach, and G.W. Kirby (eds.). Progress in the Chemistry of Organic Natural Products, Vol. 37. Springer Verlag, New York.
Chisholm, M.D., Steck, W.F., Arthur, A.P., andUnderhill, E.W. 1975. Evidence for cis-11-hexadecen-1-ol acetate as a major component of the sex pheromone of the Bertha armyworm,Mamestra configurata (Lepidoptera: Noctuidae).Can. Entomol. 107:361–366.
Conner, W.E., Eisner, T., Vander Meer, R.L., Guerrero, A., Ghiringelli, D., andMeinwald, J. 1980. Sex attractant of an arctiid moth (Utetheisa ornatrix): A pulsed chemical signal.Behav. Ecol. Sociobiol. 7:55–63.
Corey, E.J., andSuggs, J.W. 1975. Pyridinium chlorochromate. An efficient reagent for oxidation of primary and secondary alcohols to carbonyl compounds.Tetrahedron Lett. 2647–2650.
Corey, E.J., andVenkateswarlu, A. 1972. Protection of hydroxyl groups astert-butyldimethylsilyl derivatives.J. Am. Chem. Soc. 94:6190–6191.
Dale, J.A., Dull, D.L., andMosher, H.S. 1969. α-Methoxy-α-trifluoromethylphenylacetic acid, a versatile reagent for the determination of enantiomeric composition of alcohols and amines.J. Org. Chem. 9:2543–2549.
Hansen, K., Schneider, D., andBoppre, M. 1983. Chiral pheromone and reproductive isolation between the gypsy and nun moth.Naturwissenschaften 70:466–467.
Heath, R.R., Tumlinson, J.H., Leppla, N.C., McLaughlin, J.R., Dueben, B., Dundulis, E., andGuy, R.H. 1983. Identification of a sex pheromone produced by velvetbean caterpillar moth.J. Chem. Ecol. 9:645–656.
Hill, A.S., andRoelofs, W.L. 1981. Sex pheromone of the saltmarsh caterpillar moth,Estigmene acrea.J. Chem. Ecol. 7:655–668.
Hill, A.S., Kovalev, B.G., Nikolaeva, L.N., andRoelofs, W.L. 1982. Sex pheromone of the fall webworm moth,Hyphantria cunea.J. Chem. Ecol. 8:383–396.
Hodges, R.W., et al. 1983. Check List of the Lepidoptera of America North of Mexico. E.W. Classey Ltd. and The Wedge Entomological Foundation, London.
Huang, W., Pulaski, S.P., andMeinwald, J. 1983. Synthesis of highly unsaturated insect pheromones: (Z,Z,Z)-1,3,6,9-heneicosatetraene and (Z,Z,Z)-1,3,6,9-nonadecatetraene.J. Org. Chem. 48:2270–2274.
Leonhardt, B.A., Neal, J.W., Jr., Klun, J.A., Schwarz, M., andPlimmer, J.R. 1983. An unusual lepidopteran sex pheromone system in the bagworm moth.Science 219:314–316.
Mori, K., andEbata, T. 1981. Synthesis of optically active pheromones with an epoxy ring. (+)-disparlure and the saltmarsh caterpillar moth pheromone [(Z,Z)-3,6-cis-9,10-epoxyheneicosadiene].Tetrahedron Lett. 22:4281–4282.
Parham, W.E., andAnderson, E.L. 1948. The protection of hydroxyl groups.J. Am. Chem. Soc. 70:4187–4189.
Roelofs, W.L. 1979. Pages 272–285,in R.L. Rabb and G.G. Kennedy (eds.). Movements of Highly Mobile Insects: Concepts and Methodology in Research. North Carolina State University, Raleigh.
Roelofs, W.L., Hill, A.S., Linn, C.E., Meinwald, J., Jain, S.C., Herbert, H.J., andSmith, R.F. 1982. Sex pheromone of the winter moth, a geometrid with unusually low temperature precopulatory responses.Science 217:657–659.
Sendega, R.V., Prib, O.Z., andVizgert, R.V. 1968. Synthesis and alkylating properties of some unsaturated esters of arene sulfonic acids.Zh. Org. Khim. 4:1907–1909.
Sharpless, K.B., andVerhoeven, T.R. 1979. Metal-catalyzed, highly selective oxygenations of olefins and acetylenes with tert-butyl hydroperoxide. Practical considerations and mechanisms.Aldrichimica Acta 12:63–74.
Steck, W., Underbill, E.W., andChisholm, M.D. 1982. Structure-activity relationships in sex attractants for North American noctuid moths.J. Chem. Ecol. 8:731–754.
Tamaki, Y., Sugie, H., Osakabe, M., andSonnet, P. 1983. Biological activities ofR- andS-10-methyldodecyl acetates, the chiral component of the sex pheromone of the smaller tea tortrix moth (Adoxophyes sp., Lepidoptera: Tortricidae).Appl. Entomol. Zool. 18:292–294.
Underhill, E.W., Palaniswamy, P., Abrams, S.R., Bailey, B.K., Steck, W.F., andChisholm, M.D. 1983. Triunsaturated hydrocarbons, sex pheromone components ofCaenurgina erechtea.J. Chem. Ecol. 9:1413–1423.
Wong, J.W., Palaniswamy, P., Underhill, E.W., Steck, W.F., andChisholm, M.D. 1984a. Novel sex pheromone components from the fall cankerworm moth,Alsophila pometaria.J. Chem. Ecol. 10:463–473.
Wong, J.W., Palaniswamy, P., Underhill, E.W., Steck, W.F., andChisholm, M.D. 1984b. Sex pheromone components of the fall cankerworm moth,Alsophila pometaria: Synthesis and field trapping.J. Chem. Ecol. 10:1579–1596.
Author information
Authors and Affiliations
Additional information
Issued as NRCC No. 24314.
Rights and permissions
About this article
Cite this article
Wong, J.W., Underhill, E.W., MacKenzie, S.L. et al. Sex attractants for Geometrid and Noctuid moths. J Chem Ecol 11, 727–756 (1985). https://doi.org/10.1007/BF00988302
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00988302