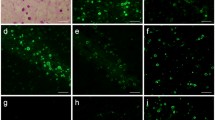
Abstract
Corpora amylacea (CA) are one of the conspicuous features of brain tissue in normal aging and neurodegenerative diseases. Quantitative protein determination of purified CA revealed a protein content of about 4% of total weight. Qualitative protein analysis revealed a broad range of polypeptides, with four being more abundant. High performance liquid chromatography (HPLC), fractionation of this protein material showed four peaks which are related to the four major polypeptides with molecular weights of 24 KD, 42 KD, 94 KD, and 133 KD. Amino acid content analysis of the 24 KD, 42 KD and 94 KD polypeptides indicated that distinct protein species are involved. N-terminal amino acid sequence analysis of the 24 KD and 42 KD polypeptides revealed in both cases homology with the N-terminal sequence of human ubiquitin.
Similar content being viewed by others
References
Anzil, A. P., Herrlinger, H., Blinzinger, K., and Kronski, D. 1974. Intraneuritic Corpora amylacea. Virchows Arch. A. Path. Anat. Histol. 364:297–304.
Ramsey, H. J. (1965). Ultrastructure of Corpora amylacea. J. Neuropathol. Exp. Neurol. 24:25–39.
Palmucci, L., Anzil, A. P., and Luh, S. (1982). Intra-astrocytic glycogen granules and Corpora amylacea stain positively for polyglucosans: A cytochemical contribution on the fine structural polymorphism of particulate polysaccharide. Acta Neuropathol. (Berl), 57:99–102.
Alder, N. (1953). On the nature, origin and distribution of the Corpora amylacea of the brain with observation on some new staining reactions. Br. J. Psychiat., 99:689–697.
Averback, P. (1981). Parasynaptic Corpora amylacea in the striatum. Arch. Pathol. Lab. Med., 105:334–335.
Ellis, R. T. (1920). Norms for some structural changes in the human cerebellum from birth to old age. The J. Comp. Neurol., 32:1–33.
Fleming, P. D., Cordoza, M. E., Woods, S. G., Griesbach, E. J. and Worcester, M. A. (1987). Corpora amylacea increased in Alzheimer's disease. Neurology (suppl. 1), 37:157.
Robitaille, Y., Carpenter, S., Karpati, G. and DiMauro, S. (1980). A distinct form of adult polyglucosan disease with massive involvement of central and peripheral neuronal processes and astrocytes. Brain, 103:315–336.
Fleming, P. D. and Rogers, J. (1986) Neuropathology of olfactory system in Alzheimer's disease and normal aging. Soc. Neurosci. Abstr., 12:1314.
Roukema, P. A., and Oderkerk, C. H. (1970). Isolation and preliminary characterization of Corpora amylacea from human brains. Psychiat. Neurol. Neurochir. 73:87–89.
Stam, F. C. and Roukema, P. A. (1973) Histochemical and biochemical aspects of Corpora amylacea. Acta Neuropathol. (Berl), 25:95–102.
Sakai, M., Austin, J., Witmer, F. and Trueb, L. (1969a). Studies of Corpora amylacea. I: Isolation and preliminary characterization by chemical and histochemical techniques. Arch. Neurol., 21:526–544.
Sakai, M., Austin, J., Witmer, F. and Trueb, L. (1969b) Corpora amylacea: Isolation, characterization and significance. Trans. Am. Neurol. Assoc., 94:336–338.
Steyaert, A., Cissé, S., Merhi, Y., Kalbakji, A., Reid, N., Gauvreau, D. and Lacoste-Royal, G. (1990). Purification and polypeptide composition of corpora amylacea from aged human brain. J. Neurosci. Meth., 31:59–64.
Selkoe, D. J. (1986). Altered structural proteins in plaques and tangles: What do they tell us about the biology of Alzheimer's disease? Neurobiol. Aging 7:425–432.
Selkoe, D. J. (1989). Biochemistry of altered brain proteins in Alzheimer's disease. Ann. Rev. Neurosci., 12:463–490.
Lew, E. O., Rozdilsky, B., Munoz, D. G. and Perry, G. (1989). A new type of neuronal cytoplasmic inclusion: histological, ultrastructural, and immunochemical studies. Acta Neuropathol., 77:599–604.
Perry, G. (1987). Alterations of the neuronal cytoskeleton in Alzheimer disease. (G. Perry, ed.) p. 229, Plenum Press, New York.
Zhang, H., Sternberger, N. H., Rubinstein, L. J., Herman, M. M., Binder, L. I., and Sternberger, L. A. 1989. Abnormal processing of multiple proteins in Alzheimer disease. Proc. Natl. Acad. Sci. USA 86:8045–8049.
Weidmann, A., König, G., Bunke, D., Fischer, P., Salbaum, J. M., Masters, C. L., and Beyreuther, K. (1989). Identification, biogenics, and localization of precursors of Alzheimer's disease A4 amyloid protein. Cell, 57:115–126.
Lowe, J., Blanchard, A., Morrell K., Lennox, G., Reynolds, L., Billett, M., Landon, M. and Mayer, R. J. (1988). Ubiquitin is a common factor in intermediate filament inclusion bodies of diverse type in man, including those of Parkinson's disease, Pick's disease and Alzheimer's disease, as well as Rosenthal fibers in cerebellar astrocytics, cytoplasmic bodies in muscle and Mallory bodies in alcoholic liver disease. J. Pathol., 155:9–15.
Manetto, V., Perry, G., Tabaton, M., Mulvihill, P., Fried, V. Smith, H., Gambetti, P., and Autilio-Gambetti, L. (1988) Ubiquitin is associated with abnormal cytoplasmic filaments characteristic of neurodegenerative diseases. Proc. Natl., Acad. Sci, USA, 84:4501–4505.
Manetto, V., Abdul-Karim, F. W., Perry, G., Tabaton, M., Autilio-Gambetti, L., and Gambetti, P. (1989). Selective presence of ubiquitin intracellular inclusions. Ann. J. Pathol. 134,3:505–513.
Power, D. M., Hue, D., Brion, J. P., Kahn, J., and Anderton, B. H. (1988). Neuronal inclusion in Alzheimer's, Parkinson's and Pick's diseases. Neurosci. Lett., 22:S55.
Cole, G. M., and Timiras, P. S. (1987) Ubiquitin-protein conjugates in Alzhimer's lesions. Neurosci. Lett. 79:207–212.
Perry, G., Mulvihill, P., Pried, V. A., Smith, H. T., GrundkeIqbal, I., and Iqbal, K. 1989. Immunochemical properties of ubiquitin conjugates in the paired helical filaments of Alzheimer's disease. J. Neurochem, 52:1523–1528.
Goldstein, G., Sheid, M., Hammerling, V., Boyse, E. A., Schlesinger, D. M., and Nail, H. D. 1975. Isolation of polypeptide that has lymphocyte differentiating properties and is probably represented universally in living cells. Proc. Natl. Acad. Sci. USA, 72:11–15.
Finley, D., and Varshavsky, A. (1985). The ubiquitin system: Fonctions and mechanisms. Trends Biochem. Sci., 10:343–346.
Hershko, A., and Ciechanover, A. (1986). The ubiquitin pathway for the degradation of intracellular proteins. Prog. Nucleic Acid Res. Mol. Biol., 33:19–56.
Hershko, A., Ciechanover, A., Heller, H., and Haasarose, I.A. (1980). Proposed role of ATP in protein breakdown: Conjugation of proteins with multiple chains of polypeptide ATP-dependant proteolysis. Proc. Natl. Acad. Sci. USA, 77:1783–1786.
Dice, J.F. (1987). Molecular determinants of protein half-lives in eukaryotic cells. FASEB, J., 1:349–357.
Busch, M., and Goldnopf, I. L. (1982). Ubiquitin-protein conjugates. Mol. Cell. Biochem., 40:173–187.
St-John, T., Gallatin, W. M., Siegelman, M., Smith, H. T., Fried, V. A., and Weissman, I. L. (1986). Expression, cloning of a lymphocyte homing receptor cDNA: Ubiquitin is the reactive species. Science, 231:845–850.
Siegelman, M., Bond, M. W., Gallatin, MW, St-John, T., Smith, H. T., Fried, V. A., and Weissman, I. L. (1986). Cell surface molecule associated with lymphocyte homing is ubiquitin branchedchain glycoprotein. Science, 231:823–829.
Murti, K. G., Smith, H. T., and Fried, V. A. 1988. Ubiquitin is a component of the microtule network. Proc. Natl. acad. Sci. USA, 85:3019–3023.
Ball, E., Karlik, C. C., Beall, C. J., Saville, D. L., Saparrow, J. C., Bullard, B., and Fryberg E. A. (1987). Arthrin, a myofibrillar protein of insect flight muscle, is actin-ubiquitin conjugate. Cell, 51:221–228.
Yarden, Y., Escobedo, J. A., Kuang, W. J., Yang-Feng, T. L., Daniel, T. D., Tremble, P. M., Chen, E. Y., Ando, M. E., Harkins, R. N., Francke, U., Fried, V. A., Ullrich, A., and Williams, L. T. (1986). Structure of receptor for platelet-derived growth factor helps define a family of related growth factor receptor. Nature, 323:226–232.
Laemmli, U.K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227:680–685.
Schlesinger, D. H., and Goldstein, G. (1975). Ubiquitin: Human, complete sequence with experimental details. Nature, 255:423–424.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cissé, S., Lacoste-Royal, G., Laperrière, J. et al. Ubiquitin is a component of polypeptides purified from corpora amylacea of aged human brain. Neurochem Res 16, 429–433 (1991). https://doi.org/10.1007/BF00965562
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00965562