Abstract
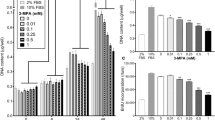
The effect of mevastatin and mevinolin on the fusion of L6 myoblasts was studied. Both compounds were potent inhibitors of myoblast fusion at concentrations as low as 0.25 μM, but fusion was restored when the inhibitors were removed. Both compounds resulted in decreased binding of conA and WGA to cell surface oligosaccharides showing they were causing a reduction in N-linked cell surface glycoproteins. There was a reduction in creatine phosphokinase activities in the presence of both compounds showing that they were affecting biochemical differentiation. The presence of both compounds inhibited the incorporation of labeled mannose from GDP-mannose into lipid-sugar and N-linked glycoprotein, but the inhibition was reversed by addition of exogenous dolichol phosphate to the incorporation mixture. The main conclusion from these studies is that mevinolin and mevastatin are inhibiting myoblast fusion by affecting the synthesis of fusogenic cell surface N-linked glycoproteins probably by affecting the synthesis of dolichol phosphate containing oligosaccharides that are required as intermediates in N-linked glycoprotein biosynthesis.
Similar content being viewed by others
Abbreviations
- HMG-CoA:
-
3-hydroxy-3-methylglutaryl coenzyme A
- Dol:
-
dolichol
- Dol-P:
-
dolichol phosphate
- Man:
-
mannose
- GlcNAc:
-
N-acetylglucosamine
- Glc:
-
glucose
- conA:
-
concanavalin A
- WGA:
-
wheat germ agglutinin
- CPK:
-
creatine phosphokinase
References
Knudsen KA, Horwitz AF: Tandem events in myoblast fusion. Dev Biol 58: 328–338, 1977
Karlin A: Pathogenesis of the muscular dystrophies In: LP Rowland (ed.) Excerpta Medica, Amsterdam, 1977, p 73
Cates GA, Brickenden AM, Sanwal BD: Possible involvement of a cell surface glycoprotein in the differentiation of skeletal myoblasts. J Biol Chem 259: 2646–2650, 1984
Cates GA, Nandan D, Brikenden AM, Sanwal BD: Differentiation defective mutants of skeletal myoblasts altered in a gelatin-binding glycoprotein. Biochem Cell Biol 65: 767–775, 1987
Lev A, Holland PC: Developmental regulation of a secreted gelatin-binding protein during myogenesisin vitro. Biochem Cell Biol 65: 1031–1038, 1987
Parfett CLJ, Jamieson JC, Wright JA: A correlation between loss of fusion potential and defective formation of mannose-linked lipid intermediates in independent concanavalin A-resistant myoblast cell lines. Exp Cell Res 130: 1–14, 1981
Parfett CLJ, Jamieson JC, Wright JA: Changes in cell surface glycoproteins on non-differentiating L6 rat myoblasts selected for resistance to concanavalin A. Exp Cell Res 144: 405–415, 1983
Spearman MA, Jamieson JC, Wright JA. Studies on the effect of glycoprotein processing inhibitors on fusion of L6 myoblast cell lines. Exp Cell Res 168: 116–126, 1987
Wayne S, Jamieson JC, Spearman MA, Wright JA: Studies on the effect of ketoconazole on the fusion of L6 myoblasts. Mol Cell Biochem 92: 137–146, 1990
Gupta A, Sexton RC, Rudney H: Modulation of regulatory oxysterol formation and low density lipoprotein suppression of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase activity by ketoconazole. A role for cytochrome P450 in the regulation of HMG-CoA reductase in rat intestinal epithelial cells. J Biol Chem 261: 8348–8356, 1986
Trzaskos JM, Fischer RT, Favata MF: Mechanistic studies of lanosterol C-32 demethylation. Conditions which promote oxysterol intermediate accumulation during the demethylation process. J Biol Chem 261: 16937–16942, 1986
Kraemer FB, Spilman SD: Effects of ketoconazole on cholesterol synthesis. J Pharm Exp Therap 238: 905–911, 1986
Endo A, Kuroda M, Tsujita Y: ML-236A, ML-236B and ML-236C, new inhibitors of cholesterogenesis produced bypenicillium citrinum. J Antibiot (Japan) 29: 1346–1348, 1976
Hardeman EC, Endo A, Simoni RD: Effects of compactin on the levels of 3-hydroxy-3-methylglutaryl coenzyme A reductase in compactin-resistant C100 and wild type cells. Arch Biochem Biophys 232: 549–561, 1984
Endo A: The discovery and development of HMG-CoA reductase inhibitors. J Lipid Res 33: 1569–1582, 1992
Tanner MJA, Anstee DJ: A method for the direct demonstration of the lectin-binding components of the human erythrocyte membrane. Biochem J 153: 265–270, 1976
Wright JA, Jamieson JC, Ceri H: Studies on glycyprotein biosynthesis in concanavalin A-resistant cell lines. Defective formation of mannose-linked lipid intermediates. Exp Cell Res 121: 1–8, 1979
Jamieson JC: Glycosylation and migration of membrane and secretory proteins. In: H Glaumann, T Peters, C Redman (eds) Plasma Protein Secretion by the Liver, Chapter 10. Academic Press, New York, 1983, pp 257–284
Goldstein JL, Brown MS: Regulation of the mevalonate pathway. Nature (London) 343: 425–430, 1990
Cornell RB, Nissley SM, Horwitz AF: Cholesterol availability modulates myoblast fusion. J Cell Biol 86: 820–824, 1980
Cambron LD, Leskawa KC: Glycosphingolipids during myoblast fusion; possible role of cell surface glycosyltransferases. Glycoconjugate J 8: 163, 1991
Weinstein J, Lee EU, McEntee K, Lai P-H, Paulson JC: Primary structure of β-galactoside α2-6sialyltransferase. Conversion of membrane bound enzyme to soluble forms by cleavage of the NH2 terminal signal anchor. J Biol Chem 262: 17735–17743, 1987
Fast DG, Jamieson JC, McCaffrey G: THe role of the carbohydrate chains of Ga1β1-4GlcNAcα2-6sialyltransferase for enzyme activity. Biochim Biophys Acta (in press), 1993
Smith PF, Eydelloth RS, Grossman SJ, Stubbs RJ, Schwartz MS: HMG-CoA reductase inhibitor induced myopathy in the rat. Cyclosporin A interaction and mechanism studies. J Pharmac Exp Ther 257: 1225–1235, 1991
Nakahara K, Kuriyama M, Yoshidome H, Nagata K, Nagado T, Nagagawa M, Arimura K, Higuchi I, Osame M: Experimental simvastatin induced myopathy in rabbits. J Neurol Sci 113: 114–117, 1992
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Belo, R.S., Jamieson, J.C. & Wright, J.A. Studies on the effect of mevinolin (lovastatin) and mevastatin (compactin) on the fusion of L6 myoblasts. Mol Cell Biochem 126, 159–167 (1993). https://doi.org/10.1007/BF00925694
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00925694