Abstract
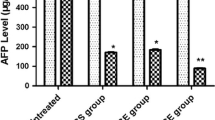
We studied the effect of transcatheter arterial injection of lymphokine-activated killer (LAK) cells in conjunction with interleukin-2 on 13 patients with liver cancers, 9 primary hepatocellular carcinomas (HCC), and 4 metastatic liver cancers. Based on the fact that the LAK-cell phenomenon was observed to exist equally in these patients as in healthy controls, an injection of autologous LAK cells into these patients was performed. Of 13 patients receiving LAK cells plus interleukin-2, one had a partial response and three had minor responses. The response duration of this “partial-response” patient was around 6 months after the LAK-cell injection. Simultaneously, a reduction of serum AFP level was found in six of eight HCC patients. One patient having a minor response after receiving this therapy had severe side effects, such as dyspnea and hypotension. The others had only a transient fever which was easy to control by medication. Thus the present study indicates that a measurable tumor regression and a reduction of serum AFP level are observed in some liver cancers after undergoing this therapy.
Similar content being viewed by others
References
Yron I, Wood TA, Spiess PJ, Rosenberg SA: In vitro growth of murine T-cells. V. The isolation and growth of lymphoid cells infiltrating syngeneic solid tumors. J Immunol 125:238–245, 1980
Lotze MT, Grimm EA, Mazumder A, Strausser JL, Rosenberg SA: Lysis of fresh and cultured autologous tumor by human lymphocytes cultured in T-cell growth factor. Cancer Res 41:4420–4425, 1981
Eberlein TJ, Rosenstein M, Rosenberg SA: Regression of a syngeneic solid tumor by systemic transfer of lymphoid cells expanded in interleukin-2. J Exp Med 156:385–397, 1982
Mulé JJ, Shu S, Schwarz SL, Rosenberg SA: Adoptive immunotherapy of established pulmonary metastases with LAK cells and recombinant interleukin-2. Science 225:1487–1489, 1984
Mazunder A, Rosenberg SA: Successful immunotherapy of natural killer-resistant established pulmonary melanoma metastases by the intravenous adoptive transfer of syngeneic lymphocytes activated in vitro by interleukin-2. J Exp Med 159:495–507, 1984
Taniguchi T, Matsui H, Fujita T,et al.: Structure and expression of a cloned cDNA for human interleukin-2. Nature 302:305–310, 1983
Adler A, Stein JA, Kedar E, Naor D, Weiss DW: Intralesional injection of interleukin-2-expanded autologous lymphocytes in melanoma and breast cancer patients: A pilot study. J Biol Resp Modif 3:491–500, 1984
Rosenberg SA: Immunotherapy of cancer by systemic administration of lymphoid cells plus interleukin-2. J Biol Resp Modif 3:501–511, 1984
Slankard-Chahinian M, Holland JF, Gordon RE, Becker J, Ohnuma T: Adoptive autoimmunotherapy. Cytotoxic effect of an autologous long-term T-cell line on malignant melanoma. Cancer 53:1066–1072, 1984
Rosenberg SA, Lotze MT, Muul LM,et al.: Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med 313:1485–1492, 1985
Rosenberg SA, Lotze MT, Muul LM,et al.: A new approach to the therapy of cancer based on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2. Surgery 100:262–271, 1986
Rosenberg SA, Lotze MT, Muul LM,et al.: A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med 316:889–897, 1987
West WH, Tauer KW, Yannelli JR,et al.: Constant-infusion recombinant interleukin-2 in adoptive immunotherapy of advanced cancer. N Engl J Med 316:898–905, 1987
Gorelik E, Kedar E, Sredni B, Herberman R: In vivo antitumor effects of local adoptive transfer of mouse and human cultured lymphoid cells. Int J Cancer 28:157–164, 1981
Lotze MT, Line BR, Mathisen DJ, Rosenberg SA: The in vivo distribution of autologous human and murine lymphoid cells grown in T cell growth factor (TCGF): Implications for the adoptive immunotherapy of tumors. J Immunol 125:1487–1493, 1980
Rosenberg SA, Eberlein TJ, Grimm EA, Lotze MT, Mazumder A, Rosenstein M: Development of long-term cell lines and lymphoid clones reactive against murine and human tumors: A new approach to the adoptive immunotherapy of cancer. Surgery 92:328–336, 1982
Shiomi S, Kuroki T, Ichiba K,et al.: Distribution of111Inoxine labeled lymphocytes on adoptive immunotherapy of hepatoma. Nippon Shokakibyo Gakkai Zasshi 84:1866, 1987
Lotze MT, Frana LW, Sharrow SO, Robb RJ, Rosenberg SA: In vivo administration of purified human interleukin-2. I. Half-life and immunologic effects of the Jurkat cell line-derived interleukin-2. J Immunol 134:157–166, 1985
Lotze MT, Matory YL, Ettinghausen SE,et al.: In vivo administration of purified human interleukin-2. II. Half-life, immunologic effects, and expansion of peripheral lymphoid cells in vivo with recombinant IL-2. J Immunol 135:2865–2875, 1985
Becker FF: Hepatoma—Nature's model tumor. A review. Am J Pathol 74:179–210 1974
Nakashima T, Okuda K, Kojiro M,et al.: Pathology of hepatocellular carcinoma in Japan. 232 consecutive cases autopsied in ten years. Cancer 51:863–877, 1983
Okuno K, Takagi H, Nakamura T,et al.: Treatment of unresectable hepatoma, via selective hepatic arterial infusion of lymphokine-activated killer cells generated from autologous spleen cells. Cancer 58:1001–1006, 1986
Ishikawa T, Imawari M, Moriyama T,et al.: Immunotherapy of hepatocellular carcinoma with autologous lymphokine-activated killer cells and/or recombinant interleukin-2. J Cancer Res Clin Oncol 114:283–290, 1988
Adoptive immunotherapy with lymphokine-activated killer cells plus recombinant interleukin-2 in patients with unresectable hepatocellular carcinoma. Hepathology 10:349–353, 1989
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Komatsu, T., Yamauchi, K., Furukawa, T. et al. Transcatheter arterial injection of autologous lymphokine-activated killer (LAK) cells into patients with liver cancers. J Clin Immunol 10, 167–174 (1990). https://doi.org/10.1007/BF00917917
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00917917