Summary
-
1.
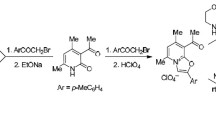
The course of aminomethylation and hydroxymethylation in the 2-alkyl-3-pyridinol series was studied with the aid of proton magnetic resonance and chemical methods.
-
2.
Methods were developed for the synthesis of 6-(aminomethyl)-[6-(hydroxymethyl)-] and 4,6-bis(dialkylammo)-2-methyl-3-pyridinols.
-
3.
The 6-mono(aminomethyl) derivative is formed at first, and this reacts further in the 4-position of the pyridine ring with formation of the 4,6-bis(dialkylaminomethyl) derivative.
-
4.
5-Hydroxy-6-methyl-2,4-pyridinedimethanol (isopyridoxine) was synthesized.
Similar content being viewed by others
Literature cited
K. M. Dyumaev, L. D. Smirnov, and V. F. Bystrov, Izv. AN SSSR, Otd. Khim. N., 883 (1962).
L. D. Smirnov, V. P. Lezina, V. F. Bystrov, and K. M. Dyumaev, Izv. AN SSSR, Otd. Khim., N. 752 (1963).
L. D. Smirnov, S. I. Sholina, K. E. Kruglyakova, and K. M. Dyumaev, Izv. AN SSSR, Otd. Khim. N., 890 (1963).
L. D. Smirnov, K. M. Dyumaev, N. I. Shuikin, and I. F. Bel'skii, Izv. AN SSSR, Otd. Khim. N., 2246 (1962).
E. B. Burlakova, V. D. Gaintseva, L. V. Slepukhina, N. G. Khrapova, and N. M. ÉmanuÉl', Dokl. AN SSSR,155, 1398 (1964).
Preobrazhenskii and É. I. Genkin, Chemistry of Organic Medicinals [in Russian], Goskhimizdat, Moscow-Leningrad (1953).
T. Urbanski, J. Chem. Soc., 1104 (1946).
A. Stempel and E. C. Buzzi, J. Amer. Chem. Soc.,71, 2969 (1949); R. F. Brown and S. I. Miller, J. Organ. Chem.,11, 388 (1946).
L. A. Perez-Medina, R. P. Mariella, and S. M. McElvain, J. Amer. Chem. Soc.,69, 2574 (1947).
Organic Reactions,1 [Russian translation], IL, Moscow (1948), p. 403.
L. M. Jackmann, Application of NMR Spectroscopy in Organic Chemistry, Pergamon Press, London (1959); J. A. Pople, W. G. Schneider, and H. J. Bernstein, High-Resolution Nuclear Magnetic Resonance [Russian translation], IL, Moscow (1962).
V. F. Bystrov, K. M. Dyumaev, V. P. Lezina, and G. A. Nikiforov, Dokl. AN SSSR,148, 1077 (1963).
V. A. Afanas'ev, V. F. Bystrov, L. L. Dekabrun, Yu. N. Kil'yanov, and A. U. Stepanyants, Zavodsk. laborat., No. 1, 102 (1962).
Author information
Authors and Affiliations
Additional information
The authors thank N. M. Émanuel for constant interest in the work.
Rights and permissions
About this article
Cite this article
Smirnov, L.D., Lezina, V.P., Bystrov, V.F. et al. Sterically hindered 3-pyridinols Communication 5. Study of the course of aminomethylation and hydroxymethylation reactions in the 2-alkyl-3-pyridinol series with the aid of proton magnetic resonance and of chemical methods. Russ Chem Bull 14, 1797–1804 (1965). https://doi.org/10.1007/BF00850162
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00850162