Summary
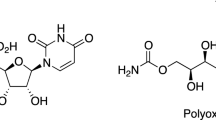
A short and economical synthesis of 3-deoxy-D-arabino-2-heptulosonic acid (4) and 3-deoxy-D-arabino-2-heptulose (kamusol,8) has been developed. In the key step of the reaction sequence, the indium mediated allylation ofD-erythrose in an aqueous solvent system was utilized generating a seven carbon backbone which was further transformed into the title compounds.
Zusammenfassung
Eine kurzer und ökonomischer Syntheseweg für 3-Desoxy-D-arabino-2-heptulosonsäure (4) und 3-Desoxy-D-arabino-2-heptulose (Kamusol,8) wurde entwickelt. Im Schlüsselschritt der Synthesesequenz wurde die indiumunterstützte Allylierung vonD-Erythrose in wäßrigen Reaktionsmedien angewendet. Das mittels dieser Methode auf sieben Kohlenstoffatome verlängerte Kohlenhydrat-Grundgerüst konnte einfach in die Titelverbindungen übergeführt werden.
Similar content being viewed by others
References
Schauer R (1982) Sialic acids. Springer Verlag, Wien
Schauer R (1982) Adv Carbohydr Chem Biochem40: 131
Unger FM (1981) Adv Carbohydr Chem Biochem38: 323
Haslam E (1974) The Shikimate Pathway. Butterworth, London
Johnson CR, Kozak J (1994) J Org Chem59: 2910
Lopez-Herrera FJ, Sarabia-Garcia F (1994) Tetrahedron Lett35: 6705
Ramage R, MacLeod AM, Rose GW (1991) Tetrahedron47: 5625
Dondoni A, Marra A, Merino P (1994) J Am Chem Soc116: 3324
Reimer LM, Conley DL, Pompliano DL, Frost JW (1986) J Am Chem Soc108: 8010
Garner CC, Herrmann KM (1984) Carbohydr Res132: 317
Sugai T, Shen G-J, Ichikawa Y, Wong C-H (1993) J Am Chem Soc115: 413
Augé C, Delest V (1995) Tetrahedron Asymm6: 863
Horton D, Nickol RG, Varela O (1987) Carbohydr Res168: 295
Banaszek A (1995) Tetrahedron51: 4231
Devianne G, Escudier J-M, Baltas M, Gorrichon L (1995) J Org Chem60: 7343
Binder WH, Prenner WH, Schmid W (1994) Tetrahedron50: 749
Prenner RH, Binder WH, Schmid W (1994) Liebigs Ann Chem 73
Chen T-H, Lee M-C (1995) J Org Chem60: 4228
Chen T-H, Li C J (1992) J Chem Soc Chem Commun: 747
Gordon DM, Whitesides GM (1993) J Org Chem58: 7937
Gao J, Härter R, Gordon DM, Whitesides GM (1994) J Org Chem59: 3714
Kim E, Gordon DM, Schmid W, Whitesides GM (1993) J Org Chem58: 5500
Cintas P (1995) Synlett: 1087
Perlin AS (1962) Methods Carbohydr Chem1: 64
Villieras J, Rambaud M (1987) Org Synth66: 220
Kamal A, Haider Y, Akhtar R, Quereshi AS (1971) Pak J Sci Ind Res14: 63; (1971) Chem Abstr75: 126859d
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Prenner, R.H., Schmid, W. Indium mediated allylation in carbohydrate chemistry: A convenient synthesis of 3-deoxy-D-arabino-2-heptulosonic acid (DAH) and 3-deoxy-D-arabino-2-heptulose (kamusol). Monatsh Chem 127, 1045–1050 (1996). https://doi.org/10.1007/BF00807577
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00807577