Summary
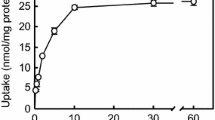
Chloroquine is an antimalarial and antirheumatic lysosomotropic drug which inhibits taurine uptake into and increases efflux from cultured human lymphoblastoid cells. It inhibits taurine uptake by rat lung slices and affects the uptake and release of cystine from cystinotic fibroblasts. Speculations on its mode of action include a proton gradient effect, a non-specific alteration in membrane integrity, and membrane stabilization. In this study, the effect of chloroquine on the uptake of several amino acids by rat renal brush border membrane vesicles (BBMV) was examined. Chloroquine significantly inhibited the secondary active, NaCl-dependent component of 10µM taurine uptake at all concentrations tested, but did not change equilibrium values. Analysis of these data indicated that the inhibition was non-competitive. Taurine uptake was reduced at all osmolarities tested, but inhibition was greatest at the lowest osmolarity. Taurine efflux was not affected by chloroquine, nor was the NaCl-independent diffusional component of taurine transport. Chloroquine (1 mM) inhibited uptake of the imino acids L-proline and glycine, and the dibasic amino acid L-lysine. It inhibited the uptake of D-glucose, but not the neutralα-amino acids L-alanine or L-methionine. Uptake of the dicarboxylic amino acids, L-glutamic acid and L-aspartic acid, was slightly enhanced. With regard to amino acid uptake by BBMV, these findings may support some of the currently proposed mechanisms of the action of chloroquine but further studies are indicated to determine why it affects the initial rate of active amino acid transport.
Similar content being viewed by others
References
Booth AG, Kenny AJ (1974) A rapid method for the preparation of microvilli from rabbit kidney. Biochem J 142: 575–581
Bradford M (1976) A rapid and sensitive method for the quantitiation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–253
Chesney RW (1987) New functions for an old molecule. Pediatr Res 22: 755–759
Chesney RW, Sacktor B, Rowen RR (1973) The binding of D-glucose to the isolated rabbit renal brush border. J Biol Chem 218: 2182–2191
Chesney RW, Gusowski N, Friedman AL (1983) Renal adaptation to altered dietary sulfur amino acid intake occurs at the luminal brush border membrane. Kidney Int 24: 588–594
Chesney RW, Gusowski N, Dabbagh S (1985) Renal cortex taurine content regulates renal adaptive response to altered dietary intake of sulfur amino acids. J Clin Invest 76: 2213–2221
Chesney RW, Gusowski N, Padilla M, Lippincott S (1986) Effect of amino acid intake on brush-border membrane uptake of sulfur amino acids. Am J Physiol 251: F125-F131
Chesney RW, Zelikovic I, Budreau A, Randle D (1991) Chloride and membrane potential dependence of sodium ion-proline symport. J Am Soc Nephrol 2: 885–893
Colombo MI, Bertini F (1988) Properties of binding sites for chloroquine in liver microsomal membranes. J Cell Physiol 137: 598–602
Daniel WD (1983) Biostatistics: a foundation for analysis in the health sciences. Wiley, New York, pp 177–180
Danpure CJ, Jennings PR, Fyfe DA (1986) Further studies on the effect of chloroquine on the uptake, metabolism and intracellular translocation of [35S]-cystine in cystinotic fibroblasts. Biochim Biophys Acta 885: 256–265
DeGroot PG, Elferink R, Hollemans M, Strijland A, Westerveld A, Meera Khan P, Tager JM (1981) Inactivation by chloroquine ofα-galactosidase in cultured human skin fibroblasts. Exp Cell Res 136: 327–333
Dixon TF, Purdom M (1954) Serum 5-nucleotidase. J Clin Pathol 7: 341–343
Fass SJ, Hammerman MR, Sacktor B (1977) Transport of amino acids in renal brush border membrane vesicles. J Biol Chem 252: 583–590
Go Ml, Lee HS (1990) Effects of mefloquine on the release of marker enzymes from the rat liver crude lysosomal fraction. Jpn J Pharmacol 53: 195–199
Goodman LS, Gilman AG (1990) The pharmacological basis of therapeutics, 8th edn. In: Gilman AG, Rall TW, Nies AS, Taylor P (eds) Pergamon Press, New York, p 983
Kotal P, Kotyk A, Jirsa M, Kordac V (1988) Effect of chloroquine on membrane permeability in yeast-release of cellular coprophyrin. Int J Biochem 20: 539–542
Leaback DH, Walker PG (1961) Studies on glucosaminidase IV. The fluorimetric assay of N-acetyl-β-D-glucosaminidase. Biochem J 78: 151–156
Lewis CPL, Cohen GM, Smith LL (1990) The identification and characterization of an uptake system for taurine into rat lung slices. Biochem Pharmacol 39: 431–437
Lowry OH, Passonneau JV (1972) A flexible system of enzymatic analysis. Academic Press, New York, p 15
Lynch AM, McGivan JD (1987) Evidence for a single common Na+-dependent transport system for alanine, glutamine, leucine and phenylalanine in brush-border membrane vesicles from bovine kidney. Biochim Biophys Acta 899: 176–184
MacIntyre AC, Cutler DJ (1988) Lysosomes in hepatic accumulation of chloroquine. J Pharm Sci 77: 196–199
McAbee DD, Clarke BL, Oka JA, Weigel PH (1990) The surface activity of the same subpopulation of galactosyl receptors on isolated rat hepatocytes is modulated by colchicine, monensin, ATP depletion and chloroquine. J Biol Chem 265: 629–635
Ohkum S, Poole B (1978) Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci USA 75: 3327–3331
Parczyk K, Kondor-Koch C (1989) The influence of pH on vesicular traffic to the surface of the polarized epithelial cell, MDCK. Eur J Cell Biol 48: 353–359
Pillion DJ, Jeske AH, Leibach FH (1976) Gamma-glutamyl transpeptidase in the urine from an isolated rabbit kidney perfused with and without DMSO. Biochem Pharmacol 25: 913–918
Post RL, Sen AK (1967) Sodium and potassium-stimulated ATPase, energy-linked reactions. Methods Enzymol 10: 762–768
Schmidt R, Sieghart W, Karokath M (1975) Taurine uptake in synaptosomal fractions of rat cerebral cortex. J Neurochem 25: 5–9
States B, Lee J, Segal S (1983) Effect of chloroquine on handling of cystine by cystinotic fibroblasts. Metabolism 32: 272–278
Strange K (1992) Regulation of solute and water balance and cell volume in the central nervous system. J Am Soc Nephrol 3: 12–27
Tallan HH, Schneidman K (1984) Taurine release from cultured human lymphoblastoid cells. Fed Proc 43: 1779
Tallan HH, Jacobson E, Wright CE, Schneidman K, Gaull GE (1983) Taurine uptake by cultured human lymphoblastoid cells. Life Sci 33: 1853–1860
Thierry J, Poujeol P, Ripoche P (1981) Interactions between Na+-dependent uptake of D-glucose, phosphate and L-alanine in rat renal brush border membrane vesicles. Biochim Biophys Acta 647: 203–210
Thoene JG, Lemons R (1980) Modulation of intracellular cystine content of cystinotic fibroblasts by extracellular albumin. Pediatr Res 14: 785–787
Tisdale HD (1967) Preparation and properties of succinic cytochrome c reductase (Complex II–III). Methods Enzymol 10: 213–217
Tratchman H (1991) Cell volume regulation: a review of cerebral adaptive mechanisms and implications for chemical treatment of osmolal disturbances. Pediatr Nephrol 5: 743–750
Tsai WP, Nara PL, Kung HF, Droszlau S (1990) Inhibition of human immuno deficiency virus infectivity by chloroquine. AIDS Res Hum Retroviruses 6: 481–489
Vorum H, Jessen H, Jørgensen KE, Sheikh MI (1988) Mechanism of transport of L-alanine by luminal-membrane vesicles from pars recta of rabbit proximal tubule. FEBS 227: 35–38
Wibo M, Poole B (1974) The uptake of chloroquine by rat fibroblasts and the inhibition of cellular protein degradation and cathepsin B1. J Cell Biol 63: 430–440
Zelikovic I, Chesney RW (1989) Sodium-coupled amino acid transport in renal tubules. Kidney Int 36: 351–359
Zelikovic I, Stejskal-Lorenz E, Lohstroh P, Budreau A, Chesney RW (1989) Anion dependence of taurine transport by rat renal brush border membrane vesicles. Am J Physiol 256: F646-F655
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chesney, R.W., Budreau, A.M. Chloroquine, a novel inhibitor of amino acid transport by rat renal brush border membrane vesicles. Amino Acids 8, 141–158 (1995). https://doi.org/10.1007/BF00806488
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00806488