Summary
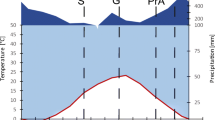
The sedgeEriophorum vaginatum in an interior Alaskan muskeg site produced leaves sequentially at about 1.5-month intervals. Each leaf remained active for two growing seasons. Young leaves (even those initiated late in the season) always had high concentrations of N, P, K and Mg and were low in Ca. Stems had high concentrations of nutrients, sugar, amino acid N and soluble organic P in autumn and spring but low concentrations in summer. Growth of leaves in spring was strongly supported by translocation from storage. Leaves approached their maximum nutrient pool before nutrient uptake began in late spring, one month before maximum biomass. Retranslocation of nutrients from aging leaves could support nutrient input into new, actively growing leaves as a consequence of the sequential leaf development. For instance retranslocation from aging leaves accounted for more than 90 and 85% of P and N input to new leaves appearing in early summer and 100% to leaves that appeared later. Leaching losses were negligible. Half time for decay of standing dead litter was 10 years. We suggest that sequential leaf development paired with highly efficient remobilization of nutrients from senescing leaves enables plants to recycle nutrients within the shoot and minimize dependence upon soil nutrients. This may be an important mechanism enablingEriophorum vaginatum to dominate nutrient-poor sites. This may also explain why graminoids with sequential leaf production cooccur with evergreen shrubs and dominate over forbs and deciduous shrubs in nutrient-poor sites in the boreal forest (e.g., in bogs) and at the northern limit of the tundra zone.
Similar content being viewed by others
References
Alexandrova VD (1970) The vegetation of the tundra zones in the USSR and data about its productivity. In: WA Fuller, PG Kevan (eds) Productivity and conservation in northern circumpolar lands, IUCN Publication No. 16, Morges Switzerland, pp 93–114
Alexandrova VD (1982) The Arctic and Antarctic: their division into geobotanical areas. English translation, Cambridge University Press, Cambridge
Andreev VN (1966) Peculiarities of zonal distribution of the aerial and underground phytomass on the East European far north. Bot Zhur 51:1401–1411
Bell KL, Bliss LC (1977) Overwinter phenology of plants in a polar semi-desert. Arctic 30:118–121
Bliss LC (1956) A comparison of plant development in microenvironments of arctic and alpine tundras. Ecol Monogr 26:303–337
Bliss LC (1981) North American and Scandinavian tundras and polar deserts. In: Bliss LC, Heal OW, Moore JJ (eds) Tundra ecosystems: a comparative analysis, Cambridge University Press, Cambridge, pp 8–24
Britton ME (1967) Vegetation of the arctic tundra. In: HP Hansen (ed) Arctic Biology, Oregon State University Press, Corvallis, pp 67–130
Chapin FS III, Johnson DA, McKendrick JD (1980) Seasonal movement of nutrients in plants of differing growth form in an Alaskan tundra ecosystem: implications for herbivory. J Ecol 68:189–209
Chapin FS III, Kedrowski RA (1983) Seasonal changes in nitrogen and phosphorus fractions and autumn retraslocation in evergreen and deciduous taiga trees. Ecology 64:376–391
Chapin FS III, Shaver GR (1981) Changes in soil properties and vegetation following disturbance of Alaskan arctic tundra. J Appl Ecol 18:605–617
Chapin FS III, Shaver GR, Kedrowski RA (1985) Environmental controls over carbon, nitrogen and posphorus chemical fractions inEriophorum vaginatum in Alaskan tussock tundra. J Ecol (submitted)
Chapin FS III, Van Cleve K, Chapin MC (1979) Soil temperature and nutrient cycling in the tussock growth form ofEriophorum vaginatum. J Ecol 67:169–189
Chapin FS III, Van Cleve K, Tieszen LL (1975) Seasonal nutrient dynamics of tundra vegetation at Barrow, Alaska. Arct Alp Res 7:209–226
Crafts AS, Crisp CE (1971) Phloem transport in plants. Freeman, San Francisco
Dowding P, Chapin FS III, Wielgolaski FE, Kilfeather P (1981) Nutrients in tundra ecosystems. In: LC Bliss, OW Heal, JJ Moore (eds) Tundra ecosystems: a comparative analysis. Cambridge University Press, Cambridge, pp 647–683
Fetcher N, Shaver GR (1983) Life histories of tillers ofEriophorum vaginatum in relation to tundra disturbance. J Ecol 71:131–147
Goodman GT, Perkins DF (1959) Mineral uptake and retention in cotton-grass (Eriophorum vaginatum L.). Nature 184:467–468
Goodman GT, Perkins DF (1968a) The role of mineral nutrients inEriophorum communities III. Growth response to added inorganic elements in twoE. vaginatum communities. J Ecol 56:667–683
Goodman GT, Perkins DF (1968b) The role of mineral nutrients inEriophroum communities IV. Potassium supply as a limiting factor in anE. vaginatum community. J Ecol 56:685–696
Hopkins DM, Sigafoos RS (1951) Frost action and vegetation patterns on Seward Peninsula, Alaska. US Geol Surv Bull 974-C:51–100
Johnson DA, Tieszen LL (1976) Aboveground biomass allocation, leaf growth, and photosynthesis patterns in tundra plant forms in arctic Alaska. Oecologia (Berl) 24:159–173
Jonasson S (1982) Organic matter and phytomass on three north Swedish tundra sites, and some connections with adjacent tundra areas. Holarct Ecol 5:367–375
Jonasson S (1983) Nutrient content and dynamics in north Swedish shrub tundra areas. Holarct Ecol 6:295–304
Jonasson S, Sköld SE (1983) Influences of frost-heaving on vegetation and nutrient regime of polygon-patterned ground. Vegetatio 53:97–112
Kredrowski RA (1983) Extraction and analysis of nitrogen, phosphorus and carbon fractions in plant material. J Plant Nutr 6:989–1011
Kummerow J, Ellis BA, Kummerow S, Chapin FS III (1983) Spring growth of shoots and roots in shrubs of an Alaskan muskeg. Am J Bot 70:1509–1515
Langer RHM (1972) How grasses grow. Studies in biology, Vol 34. Arnold, London
Malmer N, Nihlgård B (1980) Supply and transport of mineral nutrients in a subarctic mire. In: M. Sonesson (ed) Ecology of a subarctic mire. Ecol Bull No 30, Swedish Natural Science Research Council, Stockholm, pp 63–95
McCown BH (1978) The interactions of organic nutrients, soil nitrogen, and soil temperature and plant growth and survival in the arctic environment. In: LL Tieszen (ed), Vegetation and production ecology of an Alaskan arctic tundra. Springer, Berlin Heidelberg New York Tokyo, pp 435–456
Nye PH, Tinker PB (1977) Solute movement in the soil-root system. Univ California Press, Berkeley
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44: 322–331
Robertson KP, Woolhouse WH (1984) Studies of the seasonal course of carbon uptake ofEriophorum vaginatum in a moorland habitat. 1. Leaf production and senescence. J Ecol 72:423–435
Shaver GR, Chapin FS III (1980) Response to fertilization by various plant growth forms in an Alaskan tundra: nutrient accumulation and growth. Ecology 61:662–675
Shaver GR, Chapin FS III, Gartner BL (1985) Factors limiting growth and biomass accumulation ofEriophorum vaginatum L. in Alaskan tussock tundra. J Ecol (submitted)
Small E (1972a) Photosynthetic rates in relation to nitrogen recycling as an adaptation to nutrient deficiency in peat bog plants. Can J Bot 50:2227–2233
Small E (1972b) Ecological significance of four critical elements in plants of raisedSphagnum peat bogs. Ecology 53:498–503
Tamm CO (1954) Some observations on the nutrient turn-over in a bog community dominated byEriophorum vaginatum L. Oikos 5:189–194
Tieszen LL (1972) The seasonal course of aboveground production and chlorophyll distribution in a wet arctic tundra at Barrow, Alaska. Arct Alp Res 4:307–324
Tukey HB Jr (1970) The leaching of substances from plants. Annu Rev Plant Physiol 21:305–324
Wein RW (1973) Biological flora of the British Isles:Eriophorum vaginatum L. J Ecol 61:601–615
Wein RW, Bliss LC (1973) Changes in arcticEriophorum tussock communities following fire. Ecology 54:845–852
White RG (1983) Foraging patterns and their multiplier effects on productivity of northern ungulates. Oikos 40:377–384
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jonasson, S., Stuart Chapin, F. Significance of sequential leaf development for nutrient balance of the cotton sedge,Eriophorum vaginatum L.. Oecologia 67, 511–518 (1985). https://doi.org/10.1007/BF00790022
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00790022