Summary
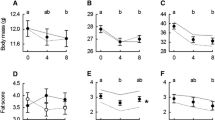
I compared the maximal aerobic metabolic rates (\(\dot V_{O_2 \max }\)), field metabolic rates (FMR), aerobic reserves (\(\dot V_{O_2 \max }\)-FMR), and basal metabolic rates (BMR) of wild and recently captured deer mice from low (440 m) and high (3800 m) altitudes. To separate the effects of the thermal environment from other altitudinal effects, I examined mice from different altitudes, but similar thermal environments (i.e., summer mice from high altitude and winter mice from low altitude). When the thermal environment was similar,\(\dot V_{O_2 \max }\), FMR, and aerobic reserve of low and high altitude mice did not differ, but BMR was significantly higher at high altitude. Thus, in the absence of thermal differences, altitude had only minor effects on the aerobic metabolism of wild or recently captured deer mice.
At low altitude, there was significant seasonal variation in\(\dot V_{O_2 \max }\), FMR, and aerobic reserve, but not BMR. BMR was correlated with\(\dot V_{O_2 \max }\), but not with FMR. The significant positive correlation of BMR with\(\dot V_{O_2 \max }\) indicates a cost of high\(\dot V_{O_2 \max }\), because higher BMR increases food requirements and energy use during periods of thermoneutral conditions.
Similar content being viewed by others
Abbreviations
- BMR :
-
basal metabolic rate
- FMR :
-
field metabolic rate
- \(P_{O_2 }\) :
-
partial pressure of oxygen
- T a :
-
ambient temperature
- T b :
-
body temperature
- T e :
-
operative temperature
- \(\dot V_{O_2 \max }\) :
-
maximal aerobic metabolic rate
References
Bakken GS (1976) A heat transfer analysis of animals: unifying concepts and the application of metabolism chamber data to field ecology. J Theoret Biol 60:337–384
Bakken GS, Santee WR, Erskine DJ (1985) Operative and standard operative temperature: tools for thermal energetic studies. Am Zool 25:933–943
Bartholomew GA (1972) Energy metabolism. In: Gordon MS (ed) Animal physiology: principles and adaptations, 2nd edn. MacMillan, New York, pp 44–72
Bartholomew GA, Vleck D, Vleck CM (1981) Instantaneous measurements of oxygen consumption during pre-flight warm-up and post-flight cooling in sphingid and saturnid moths. J Exp Biol 90:17–32
Bencowitz HZ, Wagner PD, West JB (1982) Effect of change in P50 on exercise tolerance at high altitude: a theoretical study. J Appl Physiol 53:1487–1495
Bennett AF, Ruben JA (1979) Endothermy and activity in vertebrates. Science 206:649–654
Bligh J (1973) Temperature regulation in mammals and other vertebrates. American Elsevier, New York
Casey TM (1981) Nest insulation: energy savings to brown lemmings using a winter nest. Oecologia 50:199–204
Cerretelli P (1976) Limiting factors to oxygen transport on Mount Everest. J Appl Physiol 40:658–667
Chappell MA (1984) Maximum oxygen consumption during exercise and cold exposure in deer mice,Peromyscus maniculatus. Resp Physiol 55:367–377
Chappell MA (1985) Effects of ambient temperature and altitude on ventilation and gas exchange in deer mice (Peromyscus maniculatus). J Comp Physiol B 155:751–758
Chappel MA, Hayes JP, Snyder LRG (1988) Hemoglobin polymorphisms in deer mice (Peromyscus maniculatus): physiology of beta-globin variants and alpha-globin recombinants. Evolution 42:681–688
Chappell MA, Holsclaw DS (1984) Effects of wind on thermoregulation and energy balance in deer mice (Peromyscus maniculatus). J Comp Physiol 154:619–625
Chappell MA, Snyder LRG (1984) Biochemical and physiological correlates of deer mouse alpha-chain hemoglobin polymorphisms. PNAS 81:5484–5488
Garland T Jr, Else PL (1987) Seasonal, sexual, and individual variation in endurance and activity metabolism in lizards. Am J Physiol 252:R439-R449
Gettinger RD (1984) Energy and water metabolism of freeranging pocket gophers,Thomomys bottae. Ecology 65:740–751
Grenot C, Pascal M, Buscarlet L, Francaz JM, Sellami M (1984) Water and energy balance in the water vole (Arvicola terrestris Sherman) in the laboratory and in the field (Haut-Doubs, France). Comp Biochem Physiol 78A:185–196
Hart JS (1957) Climatic and temperature induced changes in the energetics of homeotherms. Rev Can Biol 16:133–174
Hayes JP (1989) Field and maximal metabolic rates of deer mice (Peromyscus maniculatus) at low and high altitudes. Physiol Zool 62:732–744
Hayes JP, Chappell MA (1986) Effects of cold acclimation on maximum oxygen consumption during cold exposure and treadmill exercise in deer mice,Peromyscus maniculatus. Physiol Zool 59:473–481
Heimer W, Morrison P (1978) Effects of chronic and intermittent cold exposure on metabolic capacity ofPeromyscus andMicrotus. Int J Biometeor 22:129–134
Heldmaier G, Boeckler H, Buchberger A, Klaus S, Puchalski W, Steinlacher S, Wiesinger H (1986) Seasonal variation of thermogenesis. In: Heller HC, Musacchia XJ, Wang LCH (eds) Living in the Cold. Elsevier, New York, pp 361–372
Heroux O (1963) Patterns of morphological, physiological, and endorrinological adjustments under different environmental conditions of cold. Fed Proc 22:789–792
Hock RJ (1964) Physiological responses of deer mice to various native altitudes. In: Weihe WH (ed) The physiological effects of high altitude. MacMillan, New York, pp 59–72
Holleman DF, White RG, Feist DD (1982) Seasonal energy and water metabolism in free-living Alaskan voles. J Mammal 63:293–296
Karasov WH (1981) Daily energy expenditure and the cost of activity in a free-living mammal. Oecologia (Berl) 51:253–259
Koteja P (1987) On the relation between basal and maximum metabolic rate in mammals. Comp Biochem Physiol 87A:205–208
Lacy RC, Lynch CB, Lynch GR (1978) Developmental and adult acclimation effects of ambient temperature on temperature regulation of mice selected for high and low levels of nest-building. J Comp Physiol 123:185–192
Lynch GR (1973) Seasonal changes in thermogenesis, organ weights, and body composition in the white-footed mouse,Peromyscus leucopus. Oecologia 13:363–376
Nagy KA (1983) The doubly labeled water method: a guide to its use. UCLA Publications No 12-1417
Nagy KA, Seymour RS, Lee AK, Braithwaite R (1978) Energy and water budgets in free-livingAntechinus stuartii (Marsupialia. Dasyuridae). J Mammal 59:60–68
Pough FH, Andrews RM (1984) Individual and sibling-group variation in metabolism of lizards: the aerobic capacity model for the origin of endothermy. Comp Biochem Physiol 79A:415–419
Rosenmann M, Morrison P (1974) Physiological responses to hypoxia in the tundra vole. Am J Physiol 227:734–739
Rosenmann M, Morrison P (1975) Metabolic response of highland and lowland rodents to simulated high altitudes and cold. Comp Biochem Physiol 51A:523–530
Rosenmann M, Morrison P, Feist D (1975) Seasonal changes in the metabolic capacity of red-backed voles. Physiol Zool 48:303–310
SAS Institute (1985) SAS User's Guide: Statistics, 5 edn. SAS Inst Inc, Cary, NC
Sealander JA Jr (1952) The relationship of nest protection and huddling to survival ofPeromyscus at low temperature. Ecology 33:63–71
Segrem NP, Hart JS (1967) Oxygen supply and performance inPeromyscus. Can J Physiol Pharmacol 45:543–549
Shoemaker VH, Nagy KA, Costa WR (1976) Energy utilization and temperature regulation by jackrabbits (Lepus californicus) in the Mojave Desert. Physiol Zool 49:364–375
Snyder LRG, Hayes JP, Chappell MA (1988) Alpha-chain hemoglobin polymorphisms are correlated with altitude in deer mice (Peromyscus maniculatus). Evolution 42:689–697
Turek Z, Kreuzer F, Hoofd LJC (1973) Advantage or disadvantage of a decrease of blood oxygen affinity for tissue oxygen supply at hypoxia. Pflügers Arch 342:185–197
West JB (1984) Human physiology at extreme altitudes on Mount Everest. Science 223:784–788
Wickler SJ (1980) Maximal thermogenic capacity and body temperatures of white-footed mice (Peromyscus) in summer and winter. Physiol Zool 55:338–346
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hayes, J.P. Altitudinal and seasonal effects on aerobic metabolism of deer mice. J Comp Physiol B 159, 453–459 (1989). https://doi.org/10.1007/BF00692417
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00692417