Summary
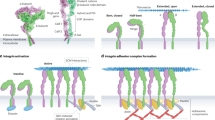
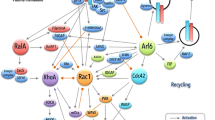
Cell motility, a primary component of tumor cell invasion, is a continuum of sequential events in which the cell extends pseudopodia, forms nascent attachments, assembles and contracts the cytoskeleton, and finally, as it translocates forward, disengages distal adhesions. What triggers cells to move? Substratum contact mediated by integrin adhesion receptors is important, but other signals such as chemokinetic factors appear to be required for continued crawling. It is now apparent that integrins do not simply bind cells to matrix in a Velcrolike fashion, but also are potent signaling molecules. Initial engagement of integrins induces their condensation into focal contacts, forming anchors to the extracellular matrix and discrete signal-transducing complexes on the cytoplasmic surface. A number of growth factors, through either autocrine or paracrine pathways, can activate the cellular machinery that mobilizes the cell. Thus, these two classes of receptors - the integrin receptors that bind specific extracellular adhesion molecules, and growth factor receptors that bind their respective ligands - can regulate cell locomotion. Not surprisingly, there is ‘cross-talk’ between integrin and growth factor receptors that occurs through their common intracellular signaling pathways. In this way, each receptor type can either amplify or attenuate the other's signal and downstream response. An example of growth factor-induced motility is the epithelial-mesenchymal transition induced by hepatocyte growth factor/scatter factor (HGF/SF). When bound to its receptor, the c-met proto-oncogene product, HGF/SF induces a phenotypic conversion that appears to be an important aspect of tumor progression in malignant carcinomas. The motogenic response produced by HGF/SF in carcinoma cells occurs in discrete steps in which integrins and focal adhesion kinase (p125FAK) are first recruited to focal contacts. This is rapidly followed by cell spreading, disruption of focal adhesions and cell-cell contacts, and, finally, cell crawling. The precise mechanism by which growth factors such as HGF/SF and its receptor induce this motogenic response and modulate integrin function has not been clearly defined but appears to involve several signaling pathways. Understanding the process by which growth factor and integrin receptors interact and regulate motility may suggest novel targets for therapeutic intervention.
Similar content being viewed by others
References
Aznavoorian S, Murphy A, Stetler-Stevenson WG, Liotta LA: Molecular aspects of tumor cell invasion and metastasis. Cancer 71: 1369–1383, 1993
Fidler IJ, Nicolson GL: The process of cancer invasion and metastasis. Cancer Bull 39: 129–131, 1987
Nicolson GL: Organ specificity of tumor metastasis: role of preferential adhesion, invasion, and growth of malignant cells at specific secondary sites. Cancer Metast Rev 7: 143–188, 1988
Hynes RO: Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69: 11–25, 1992
Sporn MB, Todaro GJ: Autocrine secretion and malignant transformation of cells. New Engl J Med 303: 878–880, 1980
Stoaker M, Ghererdi E: Regulation of cell movement: the motogenic cytokines. Biochim Biophys Acta 1072: 81–102, 1991
Lo SH, Chen LB: Focal adhesion as a signal transduction organelle. Cancer Metast Rev 13: 9–24, 1994
Juliano R: Signal transduction by integrins and its role in the regulation of tumor growth. Cancer Metast Rev 13: 25–30, 1994
Schlaepfer DD, Hanks SK, Hunter T, van der Geer P: Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature 372: 786–790, 1994
Vuori K, Ruoslahti E: Association of insulin receptor substrate-1 with integrins. Science 266: 1576–1578, 1994
Schwartz MA, Lechene C: Adhesion is required for protein kinase C-dependent activation of the Na+/H+ antiporter by PDGF. Proc Natl Acad Sci USA 89: 6138–6141, 1992
Luna EJ, Hitt AL: Cytoskeleton-plasma membrane interactions. Science 258: 955–964, 1992
Giancotti FG, Mainiero F: Integrin-mediated adhesion and signaling in tumorigenesis. Biochim Biophys Acta 1198(1): 47–64, 1994
Juliano RL, Varner JA: Adhesion molecules in cancer: the role of integrins. Curr Opin Cell Biol 5(5): 812–818, 1993
Chen J, Kim JP, Zhang K, Sarret Y, Wynn KC, Kramer R, Woodley DT: Epidermal growth factor (EGF) promotes human keratinocyte locomotion on collagen by increasing the alpha 2 integrin subunit. Exp Cell Res 209: 216–223, 1993
Stracke M, Engel J, Wilson L, Rechler M, Liotta L, Schiffman E: The type I insulin-like growth factor receptor is a motility receptor in human melanoma cells. J Biol Chem 264: 21544–21549, 1989
Klemke RL, Yebra M, Bayna EM, Cheresh DA: Receptor tyrosine kinase signaling required for integrin alpha v beta 5-directed cell motility but not adhesion on vitronectin. J Cell Biol 127(3): 859–866, 1994
Chakrabarty S: Regulation of human colon-carcinoma cell adhesion to extracellular matrix by transforming growth factor beta 1. Int J Cancer 50: 968–973, 1992
Zhang K, Matsumoto K, Douglass G, Kramer RH: Hepatocyte growth factor/scatter factor stimulates keratinocytes locomotion, migration on collagen type 1. (in preparation)
Scott G, Ewing J, Ryan D, Abboud C: Stem cell factor regulates human melanocyte-matrix interactions. Pigment Cell Res 7(1): 44–51, 1994
Sastry SK, Horwitz AF: Integrin cytoplasmic domains: mediators of cytoskeletal linkages and extra- and intracellular initiated transmembrane signaling. Curr Opin Cell Biol 5(5): 819–831, 1993
Hogervorst F, Kuikman I, Noteboom E. Sonnenberg A: The role of phosphorylation in activation of alpha6Abeta 1 laminin receptor. J Biol Chem 268: 18427–18430, 1993
Gimond C, de Melker A, Aumailley M, Sonnenberg A: The cytoplasmic domain of alpha 6A integrin subunit is anin vitro substrate for protein kinase C. Exp Cell Res 216(1): 232–235, 1995
Hemler ME: VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Ann Rev Immunol 8: 365–400, 1990
Matsumoto K, Matsumoto K, Nakamura T, Kramer RH: Hepatocyte growth factor/scatter factor induces tyrosine phosphorylation of focal adhesion kinase (p125FAK) and promotes migration and invasion by oral squamous cell carcinoma cells. J Biol Chem 269: 31807–31813, 1994
LaFlamme SE, Akiyama SA, Yamada KM: Regulation of fibronectin receptor distribution. J Cell Biol 117: 437–447, 1992
Schwartz MA, Ingber DE: Integrating with integrins. Mol Biol Cell 5(4): 389–393, 1994
Nickoloff BJ, Mitra RS, Riser BL, Dixit VM, Varani J: Modulation of keratinocyte motility. Am J Pathol 132: 543–551, 1988
Postlethwaite AE, Keski-Oja J, Moses HL, Kang AH: Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor beta. J Exp Med 165: 251–256, 1987
Muller G, Behrens J, Nussbaumer U, Bohlen P, Birchmeier W: Inhibitory action of transforming growth factor beta on endothelial cells. Proc Natl Acad Sci USA 84: 5600–5604, 1987
Matsumoto K, Lin SC, Ziober BL, Kramer RH: Receptor for HGF/SF correlates with an invasive phenotype in human breast carcinoma. Proc Am Assoc Cancer Res 35: 412a, 1995
Hay, ED: Role of cell matrix contacts in cell migration and epithelial-mesenchymal transformation. Cell Diff Dev 32: 367–376, 1990
Nagafuchi A, Shirayoshi Y, Okazaki K, Yasuda K, Takeichl M: Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature 329: 341–343, 1987
Tsarfaty I, Rong S, Resau JH, Rulong S, do Silva PP, Vande Woude GF: The met proto-oncogene: mesenchymal to epithelial cell conversion. Science 263: 98–101, 1994
Behrens J, Vakaet L, Friis R, Winterhager E, Van Roy F, Mareel M, Birchmeier W: Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/β-catenin complex in cells transformed with a temperature-sensitive v-src gene. J Cell Biol 120: 757–766, 1993
Weidner KM, Arakaki N, Hartmann G, Vanderkerchhove J, Weingart S, Rieder H, Fonatsch C, Tsubouchi C, Hishida H, Daikuhara T, Birchmeier W: Evidence for the identity of human scatter factor and human hepatocyte growth factor. Proc Natl Acad Sci USA 88: 7001–7005, 1991
Rosen EM, Nigam SK, Goldberg ID: Scatter factor and the c-met receptor: a paradigm for mesenchymal/epithelial interaction. J Cell Biol 127: 1783–1787, 1994
Cooper CS: The met oncogene: from detection by transfection to transmembrane receptor for hepatocyte growth factor. Oncogene 7: 3–7, 1992
Zarnegar R, DeFrances MC: Expression of HGF-SF in normal and malignant human tissues. EXS 65: 181–199, 1993
Sonnenberg E, Meyer D, Weidner KM, Birchmeier C: Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J Cell Biol 123: 223–235, 1993
Montesano R, Schaller G, Orci L: Induction of epithelial tubular morphogeneatsin vitro by fibroblast-derived soluble factors. Cell 66: 697–711, 1991a
Di Renzo MF, Olivero M, Ferro S, Prat M, Bongarzone I, Pilotti S, Belfiore A, Costantino A, Vigneri R, Pierotti MA,et al.: Overexpression of the c-met receptor gene in human thyroid carcinomas. Oncogene 7: 2549–2553, 1992
Di Renzo MF, Narsimhan RP, Olivero M, Bretti S, Giordano S, Medico E, Gaglia P, Zara P, Comoglio PM: Expression of the Met-HGF receptor in normal and neoplastic human tissues. Oncogene 6: 1997–2003, 1991
Di Renzo MF, Olivero M, Katsaros D, Crepaldi T, Gaglia P, Zola P, Sismondi P, Comoglio PM: Overexpression of the Met/HGF receptor in ovarian cancer. Int J of Cancer 58(5): 658–662, 1994
Ebert M, Yokoyama M, Friess H, Buchler MW, Korc M: Coexpression of the c-met proto-oncogene and HGF in human pancreatic cancer. Cancer Res 54: 5775–5778, 1994
Weidner KM, Sachs M, Birchmeier W: The met receptor tyrosine kinase transduces motility, proliferation, and morphogenic signals of scatter factor/HGF in epithelial cells. J Cell Biol 121: 145–154, 1992
Birchmeier W, Birchmeier C: Mesenchymal-epithelial transitions. Bioessays 16: 305–307, 1994
Rosen EM, Goldberg ID, Liu D, Setter E, Donovan MA, Bhargava M, Reiss M, Kacinski BM: Tumor necrosis factor stimulates epithelial tumor cell motility. Cancer Res 51: 5315–5321, 1991
Tajima H, Matsumoto K, Nakamura T: Hepatocyte growth factor has potent anti-proliferative activity in various tumor cell lines. FEBS Lett 291: 229–232, 1991
Jiang WG, Lloyds D, Puntis MCA, Nakamura T, Hallett MB: Regulation of spreading and growth of colon cancer cells by HGF. Clin Exp Metast 11: 235–242, 1993
Montesano R, Matsumoto K, Nakamura T, Orci L: Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell 67: 901–908, 1991a
Pepper MS, Matsumoto K, Nakamura T, Orci L, Montesano R: Hepatocyte growth factor increases urokinase-type plasminogen activator (u-PA) and u-PA receptor expression in Madin-Darby canine kidney epithelial cells. J Biol Chem 267: 20493–20496, 1991b
Tsarfaty I, Resau JH, Rulong S, Keydar I, Faletto DL, Vande Woude GF: The met proto-oncogene receptor and lumen formation. Science 257: 1258–1261, 1992
Kanner SB, Reynolds AB, Vines RR, Parsons JT: Monoclonal antibodies to individual tyrosine phosphorylated protein substrates of oncogene-encoded tyrosine kinases. Proc Natl Acad Sci USA 87: 3328–3332, 1990
Schaller MD, Borgman CA, Cobb BC, Reynolds AB, Parsons JT: pp125FAK, a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci USA 89: 5192–5196, 1992
Hanks SK, Calalb MB, Harper MC, Patel SK: Focal adhesion protein tyrosine kinase phosphorylated in response to cell spreading on fibronectin. Proc Natl Acad Sci USA 89: 8487–8489, 1992
Zachary I, Rozengurt E: Focal adhesion kinase (p125FAK): a point of convergence in the action of neuropeptides, integrins, and oncogenes. Cell 71: 891–894, 1992
Weiner TM, Liu ET, Craven WG: Expression of focal adhesion kinase gene and invasive cancer. Lancet 342: 1024–1025, 1993
Kornberg L, Earp HS, Parsons JT, Shaller M, Juliano RL: Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J Biol Chem 267: 23439–23442, 1992
Vuori K, Ruoslahti E: Activation of protein kinase C precedes alpha 5 beta 1 integrin mediated cell spreading on fibronectin. J Biol Chem 268: 21459–21462, 1993
Haimovich B, Lipfert L, Brugge JS, Shattil SJ: Tyrosine phosphorylation and cytoskeletal reorganization in platelets are triggered by interaction of integrin receptors with their immobilized ligands. J Biol Chem 268: 15868–15877, 1993
Liefert L, Haimovich B, Schaller MD, Cobb BS, Parsons JT, Brugge JS: Integrin-dependent phosphorylation and activation of the protein tyrosine kinase pp125FAK in platelets. J Cell Biol 119(4): 905–914, 1992
Huang MM, Lipfert L, Cunningham M, Brugge JS, Ginsberg MH: Adhesive ligand binding to integrin alphaIIb beta3 stimulates tyrosine phosphorylation of novel protein substrates prior to phosphorylation of p125FAK. J Cell Biol 122: 473–483, 1993
Rankin S, Rozengurt E: Platelet-derived growth factor modulation of focal adhesion kinase (pp125FAK) and paxillin tyrosine phosphorylation in Swiss 3T3 cells. J Biol Chem 269: 704–710, 1994
Zachary I, Sinnett-Smith J, Rozengurt E: Bombesin, vasopressin, and endothelin stimulation of tyrosine phosphorylation in Swiss 3T3 cells. J Biol Chem 267: 19031–19034, 1992
Sinnett-Smith J, Zachary I, Valverde AM, Rozengurt E: Bombesin stimulation of pp125 focal adhesion kinase tyrosine phosphorylation. J Biol Chem 268: 14261–14268, 1993
Hamawy MM, Mergenhagen SE, Siraganian RPL: Tyrosine phosphorylation of pp125FAK by aggregation of high affinity immunoglobulin E receptors requires cell adherence. J Biol Chem 268: 6851–6854, 1993
Seufferlein T, Rozengurt E: Lysophosphatidic acid stimulates tyrosine phosphorylation of focal adhesion kinase, paxillin, and p130. J Biol Chem 269: 9345–9351, 1994
Hall CL, Wang C, Lange LA, Turley EA: Hyaluronan and the hyaluronan receptor RHAMM promote focal adhesion turnover and transient tyrosine kinase activity. J Cell Biol 126: 575–588, 1994
Cantley LC, Auger KR, Carpenter C, Duckworth B, Graziani A, Kapeller R, Soltoff S: Oncogene and signal transduction. Cell 64: 281–302, 1991
Turner CE, Glenney JR, Burridge K: Paxillin: a new vinculin binding protein present in focal adhesions. J Cell Biol 111: 1059–1068, 1990
Birge RB, Fajardo JE, Reichmen C, Shoelson SE, Songyang Z: Identification and characterization of a high-affinity interaction between v-Crk and tyrosine phosphorylated paxillin in CT10-transformed fibroblasts. Mol Cell Biol 13: 4648–4656, 1993
Sabe H, Hata A, Okada M, Nakagawa H, Hanafusa H: Analysis of the binding of the Src homology 2 domain of Csk to tyrosine-phosphorylated protein in the suppression and mitotic activation of c-Src. Proc Natl Acad Sci USA 91: 3984–3988, 1994
Tanaka S, Morishita T, Hashimoto Y, Hattori S, Nakamura S, Shibuya M, Matsuoka K, Takenawa T, Kurata T, Nagashima K, Matsuda K: C3G, a guanine nucleotide-releasing protein expressed ubiquitously, bind to the src homology 3 domains of CRK and GRB2/ASH proteins. Proc Natl Acad Sci USA 91: 3443–3447, 1994
Hall A: Ras-related proteins. Curr Opin Cell Biol 5: 265–268, 1993
Khosravi-Far R, Der CJ: The ras signal transduction pathway. Cancer Metast Rev 13: 67–89, 1994
Ridley A, Paterson HF, Johnston CL, Diekmann D, Hall A: The small GTP-binding protein rac regulates growth factorinduced membrane ruffling. Cell 70: 401–410, 1992
Ridley AJ, Hall A: The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70: 389–399, 1992
Takaishi K, Sasaki T, Kato M, Yamochi W, Kuroda S, Nakamura T, Takeicji M, Takai Y: Involvement of Rho p21 small GTP-binding protein and its regulator in the HGF-induced cell motility. Oncogene 9: 273–279, 1994
Takaishi K, Kikuchi A, Kuroda S, Kotani K, Sasaki T, Takai Y: Involvement of rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI) in cell motility. Mol Cell Biol 13: 72–79, 1993
Hartmann G, Weidner KM, Schwarz H, Birchmeier W: The motility signal of scatter factor/hepatocyte growth factor mediated through the receptor tyrosine kinase met requires intracellular action of Ras. J Biol Chem 269: 21936–21939, 1994
Nobes CD, Hawkins P, Stephens L, Hall A: Activation of the small GTP-binding proteins rho and rac by growth factor receptors. J Cell Sci 108: 225–233, 1995
Riddley AJ: Membrane ruffling and signal transduction. BioEssay 16: 321–327, 1994
Hinck L, Nathke IS, Papkoff J, Nelson WJ: Dynamics of cadherin/catenin complex formation: novel protein interactions and pathways of complex assembly. J Cell Biol 125: 1327–1340, 1994
Ruoslahti E, Reed JC: Anchorage dependence, integrins, and apoptosis. Cell 77: 477–478, 1994
Meredith JE, Fazeli B, Schwartz MA: The extracellular matrix as a cell survival factor. Mol Biol Cell 4: 953–961, 1993
Frisch SM, Francis H: Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol 124: 619–626, 1994
Damsky CH, Werb Z: Signal transduction by integrin receptors for extracellular matrix: cooperative processing of extracellular information. Curr Opin Cell Biol 4: 772–781, 1992
Plantefaber LC, Hynes RO: Changes in integrin receptors on oncogenically transformed cells. Cell 56: 281–290, 1989
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Matsumoto, K., Ziober, B.L., Yao, CC. et al. Growth factor regulation of integrin-mediated cell motility. Cancer Metast Rev 14, 205–217 (1995). https://doi.org/10.1007/BF00690292
Issue Date:
DOI: https://doi.org/10.1007/BF00690292