Summary
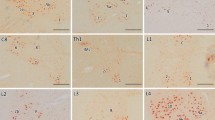
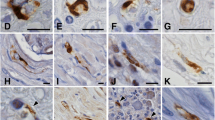
Glial bundles (GBs) in spinal nerve roots in 86 autopsy cases with various spinal lesions were examined using the peroxidase-antiperoxidase technique for glial fibrillary acidic protein (GFAP). In 19 of 22 cases of Werding-Hoffmann disease (WHD), GBs were present in the anterior roots (ARs) but absent in the youngest age group (age<1.5 months at death). GBs were numerous in classical cases (age 3–24 months), accompanying severe damage of the anterior horns and roots, but were less prominent in most cases of protracted course (age 2–8.5 years). Thus, development of GBs in the ARs of motor neuron disease at a young age seems to depend on the clinical type (age at onset and disease duration) and degree of damage to motor neurons and ARs.
Varying numbers of GBs were found also in the posterior roots (PRs) of 12 cases of WHD. In 13 patients with amyotrophic lateral sclerosis (ALS), few GBs were observed in the ARs of two and PRs of five cases without apparent relation to other clinicopathologic data. GBs in the PRs of both WHD and ALS might indicate spreading of the degenerative process to sensory neurons despite the absence of pathology detectable by routine histological stains.
Numerous GBs were found also in adults affected with polymyelitis in childhood. Varying numbers of GBs were present, however, in many different diseases, such as Friedreich ataxia, Guillain-Barré syndrome, various polyneuropathies, cervical spondylosis, ataxia telangiectasia, metachromatic leukodystrophy, and Leigh syndrome.
It is concluded that GBs are a characteristic but nonspecific reaction of the central-peripheral transition zone of the nervous system, which develops prominently in response to early damage of the myelinated fibres in the nerve roots. However, their absence in many cases of severe damage of the spinal roots renders their exact pathogenesis and significance still obscure.
Similar content being viewed by others
References
Bradley WG, Good P, Rasool CG, Adelman LS (1983) Morphometric and biochemical studies of peripheral nerves in amyotrophic lateral sclerosis. Ann Neurol 14:267–277
Chou SM, Fakadej WV (1971) Ultrastructure of chromatolytic motor neurons and anterior spinal roots in a case of Werdnig-Hoffmann disease. J Neuropathol Exp Neurol 30:368–379
Chou SM, Nonaka I (1978) Werdnig-Hoffmann disease: Proposal of a pathogenetic mechanism. Acta Neuropathol (Berl) 41:45–57
Chou SM (1979) Myeloradiculopathy of Werdnig-Hoffmann disease (WHD). A review of 43 cases. J Neuropathol Exp Neurol 38:307
Chou SM, Miike T, Eng LF (1979) Studies of Glial bundles in Werdnig-Hoffmann disease (WHD) by the immunoperoxidase method. J Neuropathol Exp Neurol 38:307
Dyck PJ, Stevens JC, Mulder DW, Espinosa RE (1975) Frequency of nerve fiber degeneration of peripheral motor and sensory neurons in amyotrophic lateral sclerosis. neurology (Minn) 25:781–785
Emery AEH (1971) The nosology of the spinal muscular atrophies. J Med Genet 8:481–495
Ghatak NR, Nochlin D (1982) Glial outgrowth along spinal nerve roots in amyotrophic lateral sclerosis. Ann Neurol 11:203–206
Ghatak NR (1983) Glial bundles in spinal nerve roots: A form of isomorphic gliosis at the junction of the central and peripheral nervous system. Neuropathol Appl Neurobiol 9:391–401
Goebel HH, Zeman W, DeMyer W (1976) Peripheral motor and sensory neuropathy of early childhood simulating Werdnig-Hoffmann disease. Neuropädiatrie 7:182–195
Griffin JW, Price DL (1980) Glial and Schwann cell responses to local axonal disease. J Neuropathol Exp Neurol 39:357
Groothuis DR, Schulman S, Wollman R, Frey J, Vick NA (1980) Demyelinating radiculopathy in Kearns-Sayre syndrome: A clinico-pathological study. Ann Neurol 8:373–380
Herpers MJHM, Budka H, McCormick D (1984) Production of glial fibrillary acidic protein (GFAP) by neoplastic cells: adaptation to the microenvironment. Acta Neuropathol (Berl) (in press)
Iwata M, Hirano A (1978) Glial bundles in the spinal cord late after paralytic anterior poliomyelitis. Ann Neurol 4:562–563
Kawamura Y, Dyck PJ, Shimono M, Okasaki H, Tateishi J, Doi H (1981) Morphometric comparison of the vulnerability of peripheral motor and sensory nerves in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 40:667–675
Maya K, Inoue K, Hirano A (1981) Pathological findings of a prolonged case of the Werdnig-Hoffmann disease. An autopsied case of 12-year-old boy. Neurol Med (Tokyo) 14:243–252 (in Japanese)
Meier C, Sollmann H (1978) Glial outgrowth and central type myelination of regenerating axons in the spinal nerve roots following transections and suture: light- and electron-microscopic study in the pig. Neuropathol Appl Neurobiol 4:21–35
Mitsumoto H, Adelman LS, Lin HS (1982) A case of congenital Werdnig-Hoffmann disease with glial bundles in spinal roots. Ann Neurol 11:214–216
Obersteiner H, Redlich E (1894) Über Wesen und Pathogenese der tabischen Hinterstrangsdegeneration. Arb Neurol Inst Wien 2:158–172
Ohama E (1982) Some aspects of anatomy and pathology of nerve roots. Adv Neurol Sci (Tokyo) 26:737–752 (in Japanese)
Raine CS, Traugott U, Stone SH (1978) Glial bridges and Schwann cell migration during chronic demyelination in the CNS. J Neurocytol 7:541–553
Shishikura K, Hara M, Sasaki Y, Misugi K (1983) A neuropathologic study of Werdnig-Hoffmann disease with special reference to the thalamus and posterior roots. Acta Neuropathol (Berl) 60:99–106
Skinneb HA (1931) Some histologic features of the cranial nerves. Arch Neurol Psychiatry 25:356–372
Smith TM, Bhawan J, Keller RB, DeGirolami U (1980) Charcot-Marie-Tooth disease associated with hypertrophic neuropathy. J Neuropathol Exp Neurol 39:420–440
Sternberger LA (1979) Immunocytochemistry, 2nd edn. Wiley, New York Chichester Brisbane Toronto
Tarlov IM (1937) Structure of the nerve root. 1. Nature of the hunction between the central and peripheral nervous system. 2. Differentiation of sensory from motor roots: Observation of mixed cranial nerves. Arch Neurol Psychiatry 37:555–583, 1338–1355
Towfighi IJ (1981) Congenital hypomyelination neuropathy: glial bundles in cranial and spinal nerve roots. Ann Neurol 10:570–573
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kimura, T., Budka, H. Glial bundles in spinal nerve roots. Acta Neuropathol 65, 46–52 (1984). https://doi.org/10.1007/BF00689827
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00689827