Summary
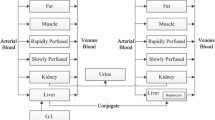
Plasma concentration-time profiles of nimustine hydrochloride, 1-[(4-amino-2-methyl-5-pyrimidinyl)methyl]-3-(2-chloroethyl)-3-nitrosourea hydrochloride (ACNU), in the mouse, rat, rabbit, and dog were determined by high-performance liquid chromatographic analysis. The pharmacokinetic parameters for these four animal species and previously reported clinical data were analyzed for investigation of interspecies correlation. Loglog plots of body weight (W; kg) vs total plasma clearance (CLtot, p; ml/min) and steady-state distribution volume (Vd, ss; 1) for the four animal species were linear, with high correlation coefficients (r 0.996 for both parameters), despite the fact that the nonrenal clearance was >97% in these species. Linear regression on the plots excluding human data yielded allometric equations (CLtot,p=50.6 W0.957; Bd, ss=1.29 W1.03) that were extrapolated to predict ACNU pharmacokinetic parameters in humans. For both parameters, however, there were 3-fold differences between the predicted and observed parametric values. To investigate these discrepancies, we measured serum protein binding of ACNU in these animal species and in humans. The values of CLtot,p and Vd,ss were converted into those of CLu tot,p and Vd,u ss, which correspond to the parameters for unbound ACNU. In this case, correlation coefficients of the log-log plots excluding human data (CLu tot,p=71.7 W0.891; Bd,u ss=1.82 W0.966) were also high (r≥0.991). The extrapolated values vs those observed in a 70-kg human were the following: CLu tot,p, 3,160 vs 2,290 ml/min; Vd,u ss, 110 vs 1061. Thus, the animal data were successfully extrapolated to yield better predictions of human pharmacokinetic parameters if the analysis was based on the unbound plasma concentration of ACNU. In addition, the predicted plasma concentration-time profile for humans also showed good agreement with the observed ones. These results suggest the importance of measuring unbound fractions of drugs for more accurate prediction of human pharmacokinetic parameters by extrapolation of animal data to the human situation.
Similar content being viewed by others
References
Boxenbaum H (1982) Interspecies scaling, allometry, physiological time, and the ground plan of pharmacokinetics. J Pharmacokinet Biopharm 10: 201
Boxenbaum H (1984) Interspecies pharmacokinetic scaling and the evolutionary-comparative paradigm. Drug Metab Rev 15: 1071
Boxenbaum H, Ronfeld R (1983) Interspecies pharmacokinetic scaling and the Dedrick plots. Am J Physiol 245: R768
Cooperative study group of phase I study on ACNU (1976) Phase I study of 1-(4-amino-2-methyl-5-pyrimidinyl)methyl-3-(2-chloroethyl)-3-nitrosourea hydrochloride (ACNU). Jpn J Clin Oncol 6: 55
Dedrick RL (1973) Animal scale-up. J Pharmacokinet Biopharm 1: 435
Hori T, Muraoka K, Saito Y, Sasahara K, Inagaki H, Inoue Y, Adachi S, Anno Y (1987) Influence of modes of ACNU administration on tissue and blood drug concentration in malignant brain tumors. J Neurosurg 66: 372
Igari Y, Sugiyama Y, Sawada Y, Iga T, Hanano M (1984) In vitro and in vivo assessment of hepatic and extrahepatic metabolism of diazepam in the rat. J Pharm Sci 73: 826
Inaba M, Kobayashi T, Tashiro T, Sakurai Y (1988) Pharmacokinetic approach to rational therapeutic doses for human tumor-bearing nude mice. Jpn J Cancer Res 70: 509
Inaba M, Tashiro T Kobayashi T, Sakurai Y, Maruo K, Ohnishi Y, Ueyama Y, Nomura T (1988) Responsiveness of human gastric tumors implanted in nude mice to clinically equivalent doses of various antitumor agents. Jpn J Cancer Res 79: 517
Inaba M, Kobayashi T, Tashiro T, Sakurai Y, Maruo K, Ohnishi Y, Ueyama Y, Nomura T (1989) Evaluation of antitumor activity in a human breast tumur/nude mouse model with a special emphasis on treatment dose. Cancer 64: 1577
McGovren JP, Williams MG, Stewart JC (1988) Interspecies comparison of acivicin pharmacokinetics. Drug Metab Dispos 16: 18
Mordenti J (1985) Man versus beast: pharmacokinetic scaling in mammals. J Pharm Sci 75: 1028
Mordenti J (1986) Dosage regimen design for pharmaceutical studies conducted in animals. J Pharm Sci 75: 852
Nishigaki T, Nakamura K, Kinoshita T, Kuwano H, Tanaka M (1985) Identification of major urinary metabolites of ACNU, 3-[(4-amino-2-methyl-5-pyrimidinyl)methyl]-1-(2-chloroethyl)-1-nitrosourea hydrochloride, in rats. J Pharmacobio-Dyn 8: 401
Nishigaki T, Nakamura K, Tanaka M (1985) In vitro metabolism of ACNU, 3-[(4-amino-2-methyl-5-pyrimidinyl)methyl]-1-(2-chloroethyl)-1-nitrosourea hydrochloride, a water-soluble antitumor nitrosourea. J Pharmacobio-Dyn 8: 409
Owens SM, Hardwick WC, Blackall D (1987) Phencyclidine pharmacokinetic scaling among species. J Pharmacol Exp Ther 242: 96
Ozawa S, Sugiyama Y, Mitsuhashi Y, Kobayashi T, Inaba M (1988) Cell killing action of cell cycle phase-non-specific antitumor agents is dependent on concentration-time product. Cancer Chemother Pharmacol 21: 185
Rowland M (1985) Physiological pharmacokinetic models and interanimal species scaling. Pharmacol Ther 29: 49
Sawada Y, Hanano M, Sugiyama Y, Iga T (1984) Prediction of the disposition of β-lactam antibioties in humans from pharmacokinetic parameters in animals. J Pharmacokinet biopharm 12: 241
Sawada Y, Hanano M, Sugiyama Y, Harashima H, Iga T (1984) Prediction of the volumes of distribution of basic drugs in humans based on data from animals. J Pharmacokinet Biopharm 12: 587
Sawada Y, Hanano M, Sugiyama Y, Iga T (1985) Prediction of the disposition of nice weak acidic and six weak basic drugs in humans from pharmacokinetic parameters in rat. J Pharmacokinet Biopharm 13: 477
Tanaka M, Nishigaki T, Nakajima S, Totsuka S, Nakamura K (1980) Distribution, excretion, and metabolism of 3-[(4-amino-2-methyl-5-pyrimidinyl)methyl]-1-(2-chloroethyl)-1-nitrosourea hydrochloride (ACNU) in rats and mice after i. v. administration. Cancer Treat Rep 64: 575
Tashiro T, Inaba M, Kobayashi T, Sakurai Y, Maruo K, Ohnishi Y, Ueyama Y, Nomura T (1989) Responsiveness of human lung cancer/nude mouse to antitumor agents in a model using clinically equivalent doses. Cancer Chemother Pharmacol 24: 187
Yamaoka K, Tanigawara Y, Nakagawa T, Uno T (1981) A pharmacokinetic analysis program (MULTI) for microcomputer. J Pharmacobio-Dun 4: 879
Yokoyama M (1980) A review on 1-(4-amino-2-methyl-5-pyrimidinyl)methyl-3-(2-chloroethyl)-3-nitrosourea hydrochloride (ACNU). Jpn J Cancer Chemother 7: 339 (in Japanese, with English summary)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mitsuhashi, Y., Sugiyama, Y., Ozawa, S. et al. Prediction of ACNU plasma concentration-time profiles in humans by animal scale-up. Cancer Chemother. Pharmacol. 27, 20–26 (1990). https://doi.org/10.1007/BF00689271
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00689271