Abstract
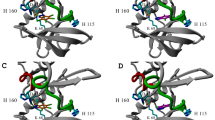
A structural refinement is proposed for the mechanistic details of the action of the serine proteases. The proposal involves ring flipping of the imidazole function of the histidine side chain as a vehicle for proton transfer. The geometric feasibility of this motion is established by molecular graphics analysis of the crystal structure ofα-chymotrypsin. It is suggested that the shape of histidine is as important as its pK a for its function at the active sites of enzymes.
Similar content being viewed by others
References
Vedani, A.; Dunitz, J. D.J. Am. Chem. Soc. 1985,107, 7653; see also Gandour, R. D.; Nabulsi, N. A. R.; Fronczek, F. R.J. Am. Chem. Soc. In press, and references therein. We thank Professor Gandour for a preprint of this manuscript.
For a review see Venkatasubban, K. S.; Schowen, R. L.Crit. Rev. Biochem. 1984,17, 1.
Sprang, S.; Standing, T.; Fletterick, R. J.; Stroud, R. M.; Finer-Moore, J.; Xuong, N.-H.; Hamlin, R.; Rutter, W. J.; Craik, C. S.Science (Washington, DC)1987,237, 905; Craik, C. S.; Roczniak, S.; Largman, C.; Rutter, W. J.id, 909; Carter, P.; Wells, J. A.Nature (London),1988,332, 564.
For alternate interpretations concerning the function of the carboxylate see Warshel, A.; Russel, S.J. Am. Chem. Soc.,1986,108, 6569; Bachovchin, W. W.Biochemistry,1986,25, 7751.
Gandour, R. D.Bioorg. Chem. 1981,10, 169.
Tsukuda, H.; Blow, D. M.; Brookhaven Data Bank; cf. Tsukada, H.; Blow, D. M.J. Mol. 1985,184, 703.
MACROMODEL 2.0 (W. C. Still, Columbia University) was used in the analysis of the active site on an Evans & Sutherland PS 390 system.
For related suggestions involving the bidentate nature of carboxylates see Knowles, J. R.; Albery, W. J.Acc. Chem. Res. 1977,10, 105 and Schepartz, A.; Breslow, R.J. Am. Chem. Soc. 1987,109, 1814.
An extensive discussion of motions elsewhere in the active sites of serine proteases is available: Gorenstein, D. G.Chem. Rev. 1987,87, 1047.
Huff, J.; Askew, B.; Duff, R. J.; Rebek, J., Jr.J. Am. Chem. Soc. 1988,110, 5908.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rebek, J. On the structure of histidine and its role in enzyme active sites. Struct Chem 1, 129–131 (1990). https://doi.org/10.1007/BF00675792
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00675792