Summary
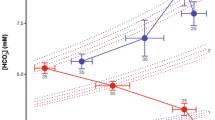
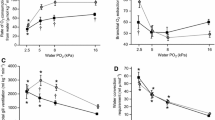
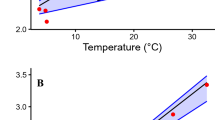
The giant salamanders of North America include 4 genera, all of which are aquatic. We have compared the efficacy of aquatic O2 uptake among them by measuring theVO2 while submerged and determining the responses to progressive hypoxia at 10–240 mmHg at 20° C. Both species ofAmphiuma were metabolic O2 conformers over the entire range ofPO2. About half ofSiren lacertina were conformers over this range, and half were regulators with an average critical O2 tension of 92 mmHg. There were no short-term changes (days) in the response ofSiren to progressive hypoxia, but one animal switched from conformation to regulation after 4–5 months. Neither genus is considered to have an exceptionally low metabolic rate. The “whole-body O2 conductance”, defined asΔVO2/ΔPO2(µl O2 · g−1 · h−1 · mmHg−1) in the range of metabolic O2 conformity, was least in the species most dependent upon air-breathing and most likely to be found in hypoxic waters (e.g., 0.076 forAmphiuma), and greatest in those that airbreathe less frequently and/or are found in relatively normoxic waters (e.g., 0.429 forNecturus). These conductances are considered to be adaptive in terms of preventing O2 loss through the skin, or in facilitating its uptake, as correlated with the O2 tensions normally prevailing in the environment of each species.
Similar content being viewed by others
References
Bentley PJ (1975) Cutaneous respiration in the congo eelAmphiuma means (Amphibia: Urodela). Comp Biochem Physiol 50A:121–124
Bond AN (1960) An analysis of the response of salamander gills to changes in the oxygen concentration of the medium. Dev Biol 2:1–20
Boutilier RG, Toews DP (1981) Respiratory properties of blood in a strictly aquatic and predominantly skin-breathing urodele,Cryptobranchus alleganiensis. Resp Physiol 46:161–176
Cagle FR (1948) Observations on a population of the salamander,Amphiuma tridactylum Cuvier. Ecology 29:479–491
Czopek J (1965) Quantitative studies on the morphology of respiratory surfaces in amphibians. Acta Anatomica 62:296–323
Gatten RE, Miller K, Full R (1991) Energetics of amphibians at rest and during locomotion. In: Feder ME, Burggren WW (eds) Environmental physiology of the amphibia. University of Chicago Press (in press)
Gehlbach FR (1973) Aestivation of the salamander,Siren intermedia. Am Midl Nat 89:455–463
Guimond RW, Hutchison VH (1972) Pulmonary, branchial and cutaneous gas exchange in the mudpuppy,Necturus maculosus maculosus (Rafinesque). Comp Biochem Physiol 42A:367–392
Guimond RW, Hutchison VH (1973a) Aquatic respiration: an unusual strategy in the hellbenderCryptobranchus alleganiensis alleganiensis (Daudin). Science 182:1263–1265
Guimond RW, Hutchison VH (1973b) Trimodal gas exchange in the large aquatic salamander,Siren lacertina (Linnaeus). Comp Biochem Physiol 46A:249–268
Guimond RW, Hutchison VH (1976) Gas exchange of the giant salamanders of North America. In: Hughes GM (ed) Respiration of amphibious vertebrates. Academic Press, New York, pp 313–338
Heisler N, Forcht G, Ultsch GR, Anderson JF (1982) Acid-base regulation in response to environmental hypercapnia in two aquatic salamanders,Siren lacertina andAmphiuma means. Resp Physiol 49:141–158
Knepton JC (1954) A note on the burrowing habits of the salamanderAmphiuma means means. Copeia 1954:69
Malvin GM, Hlastala MP (1989) Effects of environmental O2 on blood flow and diffusing capacity in amphibian skin. Resp Physiol 76:229–242
Mount RH (1975) The reptiles and amphibians of Alabama. Auburn University, Agricultural Experiment Station. Auburn, Alabama
Nickerson DM, Facey DE, Grossman GD (1989) Estimating physiological thresholds with continuous two-phase regression. Physiol Zool 62:866–887
Noble GK (1925) The integumentary, pulmonary, and cardiac modifications correlated with increased cutaneous respiration in the Amphibia: a solution of the ‘hairy frog’ problem. J Morph Physiol 40:341–416
Piiper J (1982) Respiratory gas exchange at lungs, gills and tissues: mechanisms and adjustments. J Exp Biol 100:5–22
Reno HW, Gehlbach FR, Turner RA (1972) Skin and estivational coccoon of the aquatic amphibian,Siren intermedia Le Conte. Copeia 1972:625–631
Shield JW, Bentley PJ (1973) Respiration of some urodele and anuran amphibia — I. In water, role of the skin and gills. Comp Biochem Physiol 46A:17–28
Ultsch GR (1973) The effects of water hyacinths (Eichhornia crassipes) on the microenvironment of aquatic communities. Arch Hydrobiol 72:460–473
Ultsch GR (1974) Gas exchange and metabolism in the Sirenidae (Amphibia: Candata) — I. Oxygen consumption of submerged sirenids as a function of body size and respiratory surface area. Comp Biochem Physiol 47A:485–498
Ultsch GR (1976a) Eco-physiological studies of some metabolic and respiratory adaptatons of sirenid salamanders. In: Hughes GM (ed) Respiration of amphibious vertebrates. Academic Press, New York, pp 287–312
Ultsch GR (1976b) Respiratory surface area as a factor controlling the standard rate of O2 consumption of aquatic salamanders. Resp Physiol 26:357–369
Ultsch GR, Duke JT (1990) Gas exchange and habitat selection in the aquatic salamandersNecturus maculosus andCryptobranchus alleganiensis. Oecologia 83:250–258
Viosca P (1924) A terrestrial form ofSiren lacertina. Copeia 1924 136:102–104
Wood SC, Hoyt RW, Burggren WW (1982) Control of hemoglobin function in the salamander,Ambystoma tigrinum. Mol Physiol 2:263–272
Yeager DP, Ultsch GR (1989) Physiological regulation and conformation: A BASIC program for the determination of critical points. Physiol Zool 62:888–907
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Duke, J.T., Ultsch, G.R. Metabolic oxygen regulation and conformity during submergence in the salamandersSiren lacertina, Amphiuma means, andAmphiuma tridactylum, and a comparison with other giant salamanders. Oecologia 84, 16–23 (1990). https://doi.org/10.1007/BF00665589
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00665589