Summary
-
1.
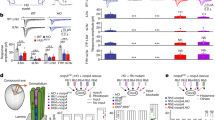
The photosensitive ‘O’ system neurons showed a graded hyperpolarization during various ‘instantaneous’ decreases in light intensity and graded depolarization during increases in light intensity. The ‘O’ system failed to respond to ‘instantaneous’ changes in light intensity of less than about 28% (Fig. 1a).
-
2.
The two motor systems, the SMNs and ‘B’ system which drive swimming muscle and tentacle contractions respectively, showed graded depolarizations and spiking frequencies during various ‘instantaneous’ decreases in light intensity (Fig. 1b, c). Increases in light intensity initially cause hyperpolarizations of these two systems. The SMNs failed to respond to ‘instantaneous’ changes in light intensity of less than about 26% (Fig. 1b).
-
3.
The ‘O’ system and SMNs respond with little habituation to repetitive changes in light intensity. Each system shows a near constant response to ‘instantaneous’ 100% changes in light intensity to all except the lowest light intensities tested (Figs. 2, 3).
-
4.
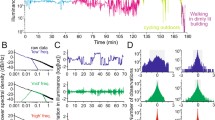
The rate of ‘O’ system hyperpolization is directly proportional to the rate of percentage decrease in light intensity (Figs. 4, 5, 7a).
-
5.
The SMNs and ‘B’ system show graded depolarizations and changes in spiking frequencies during ‘slow’, continuous decreases in light intensity. These two motor systems also show graded hyperpolizations during ‘slow’ increases in light intensity (Figs. 6, 7).
-
6.
The ‘O’ system neurons show properties common to photoreceptors in general, namely graded responses to light intensity changes and electrical coupling between adjacent cells.
Similar content being viewed by others
Abbreviations
- SMNs :
-
swimming motor neurons
References
Anderson PAV, Mackie GO (1977) Electrically coupled photosensitive neurons control swimming in a jellyfishPolyorchis. Science 197:186–188
Arkett SA (1984) Diel vertical migration and feeding behavior of a demersal hydromedusanPolyorchis penicillatus. Can J Fish Aquat Sci 41:1837–1843
Arkett SA (1985) The shadow response of a hydromedusan (Polyorchis penicillatus): behavioral mechanisms controlling diel and ontogenic vertical migration. Biol Bull 169:297–312
Arkett SA, Spencer AN (1986) Neuronal mechanisms of a hydromedusan shadow reflex. I. Identified reflex components and sequence of events. J Comp Physiol A 159:201–213
Attwell D, Wison M, Wu SM (1984) A quantitative analysis of interaction between photoreceptors in salamanders. J Physiol 352:703–738
Barlow RB (1983) Circadian rhythms int heLimulus visual system. J Neurosci 3:856–870
Baylor DA, Fuortes MGF, O'Bryan PM (1971) Receptive fields of single cones in the retina of the turtle. J Physiol 214:265–294
Fain GL (1981) Integration of spikeless neurons in the retina. In: Roberts A, Bush BMH (eds) Neurons without impulses. Cambridge Univ Press Cambridge, pp 29–60
Fain GL, Gold GH, Dowling JE (1976) Receptor coupling in the toad retina. Cold Spring Harb Symp Quant Biol 40:547–561
Fain GL, Ishida AT, Callery S (1983) Mechanisms of synaptic transmission in the retina. Vis Res 23:1239–1249
Forward RB (1977) Occurrence of a shadow response among brachyuran larvae. Marine Biol 39:331–341
Gladfelter WB (1972) Structure and function of the locomotory systems ofPolyorchis montereyensis. Helgoländer Wiss Meeresunters 23:38–79
Gold GH (1979) Photoreceptor coupling in retina of the toadBufo marinus. II. Physiology. J Neurophysiol 42:311–328
Gwilliam GF (1963) The mechanisms of the shadow reflex in Cirripedia. I. Electrical activity in the supraeosphageal ganglion and ocellar nerve. Biol Bull 125:470–485
Gwilliam GF (1965) The mechanism of the shadow reflex in Cirripedia. II. Photoreceptor cell response, 2nd order neuron responses and motor cell output. Biol Bull 129:244–256
Hisada M (1956) A study on the photoreceptor of a medusaSpirocodon saltatrix. J Fac Sci Hokkaido Univ Ser VI Zool 12:529–533
Kaplan E, Barlow RB (1980) Circadian clock inLimulus brain increases responses and decreases noise of retinal photoreceptors. Nature 286:393–395
Koike H (1983) Transmitter substance of barnacle photoreceptor. In: Grinnell AD, Moody WJ (eds) The physiology of excitable cells. AR Liss, Inc., New York, pp 523–534
Laughlin SB (1981) Neural principles in the visual system. In: Autrum H (ed) Vision in invertebrates. (Handbook of sensory physiology, vol VII/6B). Springer, Berlin Heidelberg New York, pp 133–280
Mackie GO (1975) Neurobiology ofStomotoca. II. Pacemaker and conduction pathways. J Neurobiol 6:339–378
Millecchia R, Gwilliam GF (1972) Photoreceptor in a barnacle: electrophysiology of the shadow reflex pathways inBalanus cariosus. Science 177:438–441
Mills CE (1983) Vertical migration and diel activity patterns of hydromedusae: studies in a large tank. J Plankton Res 5:619–630
Murbach L (1909) Some light reactions of the medusanGonionemus. Biol Bull 17:354–368
Negishi K, Teranishi T, Kato S (1983) Dopaminergic neurons regulate electrical and dye coupling between horizontal cells of the fish retina. In: Grinnell AD, Moody WJ (eds) The physiology of excitable cells, AR Liss, Inc, New York, pp 535–548
Ohtsu K (1983) Antagonizing effects of ultraviolet and visible light on the ERG from the ocellus ofSpirocodon saltatrix. J Exp Biol 105:417–420
Passano LM (1973) Behavioral control systems in medusae: a comparison between hydroand scyphomedusae. Publ Seto Mar Biol Lab 20:615–645
Piccolino M, Neyton J, Witkovsky P, Gershenfeld HM (1982) Gamma-aminobutyric acid antagonists decrease junctional communication between horizontal cells of the retina. Proc Natl Acad Sci 79:3671–3675
Piccolino M, Neyton J, Gerschenfeld HM (1984) Decrease of gap junction permeability induced by dopamine and cyclic adenosine 3, 5-monophosphate in horizontal cells of turtle retina. J Neurosci 4:2477–2488
Romanes GJ (1885) Jellyfish, starfish, and sea urchins. D Appleton and Co, New York
Schwartz EA (1975) Rod-rod interaction in the retina of the turtle. J Physiol 246:617–638
Schwartz EA (1976) Electrical properties of the rod syncytium in the retina of the turtle. J Physiol 257:379–400
Singla CL (1974) Ocelli of hydromedusae. Cell Tissue Res 149:413–429
Singla CL, Weber C (1982) Fine structure of the ocellus ofSarsia tubulosa. Zoomorphology 100:11–22
Sokal RR, Rohlf FJ (1969) Biometry. Freeman, San Francisco
Spencer AN (1978) Neurobiology ofPolyorchis. I. Function of effector systems. J Neurobiol 9:143–157
Spencer AN, Arkett SA (1984) Radial symmetry and the organization of central neurones in a hydrozoan jellyfish. J Exp Biol 110:69–90
Weber C (1982a) Electrical activities of a type of electroretinogram recorded from the ocellus of a jellyfish,Polyorchis penicillatus (Hydromedusa). J Exp Zool 223:231–243
Weber C (1982b) Electrical activity in response to light of the hydromedusan,Sarsia tubulosa. Biol Bull 162:413–422
Wilson JA, Phillips CE (1983) Pre-motor non-spiking interneurons. Progr Neurobiol 20:89–107
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Arkett, S.A., Spencer, A.N. Neuronal mechanisms of a hydromedusan shadow reflex. J. Comp. Physiol. 159, 215–225 (1986). https://doi.org/10.1007/BF00612304
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00612304