Abstract
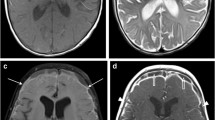
We studied 78 patients with clinically suspected central nervous system abnormalities (66 intracranial, 12 spinal) by MRI before and after adminis-tration of the nonionic contrast medium gadodiamide injection. A parallel, double-blind, randomised design was followed. Two dosages were used: 38 patients underwent studies with 0.1 mmol/kg body weight (b.w.) and 40 with 0.3 mmol/kg b. w. MRI showed abnormalities in 36 of the 38 patients receiving the lower dose and 39 of the 40 patients receiving the higher dose. In 3 patients from each group more lesions were seen following injection than before. The contrast medium improved the delineation of abnormal structures and assessment of tumour size and increased their signal intensity indices in both groups, but especially at the higher dose. Adminis-tration of gadodiamide injection provided more diagnostic information in about 75% of the patients, independently of the dose. There were no reports of discomfort, but 7 patients reported adverse events considered unrelated to the gadodiamide injection. The two doses were found to be equally safe and efficient for diagnosis.
Similar content being viewed by others
References
Kaplan GD, Aisen AM, Aravapalli SR (1991) Preliminary clinical trial of gadodiamide injection: a new nonionic gadolinium contrast agent for MR imaging. JMRI 1:57–62
Van Wagoner M, O'Toole M, Worah D, Leese PT, Quay SC (1991) A phase I clinical trial with gadodiamide injection, a nonionic magnetic resonance imaging enhancement agent. Invest Radiol 26:980–986
Greco A, McNamara MT, Lanthiez P, Quay SC, Michelozzi G (1990) Gadodiamide injection: nonionic gadolinium chelate for MR imaging of the brain and spine — Phase II–III clinical trial. Radiology 176:451–456
Sze G, Brant-Zawadzki M, Haughton VM, Maravilla KR, McNamara MT, Kumar AJ, Aisen AM, Dreisbach JN, Bradley WG Jr, Weinreb JC, Drayer BP, Tsuruda JS, Hesselink JR, Johnson CE, Zimmerman RD, Weingast GR (1991) Multicenter study of gadodiamide injection as a contrast agent in MR imaging of the brain and spine. Radiology 181:693–699
Myhr G, Rinck PA, Børscth A (1992) Gadodiamide injection and gadopentetate dimeglumine. A double-blind study in MR imaging of the CNS. Acta Radiol 33:405–409
Valk J, Algra PR, Hazenberg CJ, Slooff WBM, Svaland MG (1993) A double blind comparative study of gadodiamide injection and gadopentetate dimeglumine in MR-examinations of the CNS. Neuroradiology 35:173–177
Balériáux D, Matos C, De Greef D (1993) Gadodiamide injection as a contrast medium for MRI of the central nervous system: a comparison with gadolinium DOTA. Neuroradiology 35:490–494
Runge VM, Kaufman DM, Wood ML, Adelman LS, Jacobson S (1989) Experimental trials with Gd (DO3A) — a nonionic magnetic resonance contrast agent. Nucl Med Biol 16:561–567
Runge VM, Gelblum DY, Pacetti ML, Carolan F, Heard G (1990) Gd-HPDO3A in clinical MR imaging of the brain. Radiology 177:393–400
Yuh WTC, Fisher DJ, Engelken JD, Greene GM, Sato Y, Ryals T, Crain MR, Ehrhardt JC (1991) MR evaluation of CNS tumours: dose comparison study with gadopentetate dimeglumine and gadoteridol. Radiology 180:485–491
Runge VM, Brandley WG, Brandt-Zawadzki MN, Carvlin MJ, De Simone DN, Dean BL, Dillon WP, Drayer BP, Flanders AE, Harms SE, Haughton VM, Howieson J, Joy SE, Kanal E, Kumar AJ, Liu TH, Lufkin RB, Maravilla KR, Mezrich RS, Mikhael MA, Morgan FW, Nadel SN, Pollei SR, Pomeranz SJ, Price AC, Ramsey RG, Yuh WTC, Zelch JV (1991) Clinical safety and efficacy of gadoteridol: a study in 411 patients with suspected intracranial and spinal disease. Radiology 181:701–709
Yee H, Zin A (1971) An auto analyzer procedure for serum iron and total iron binding capacity with use of ferrozine. Clin Chem 17:950–953
Trudeau DL, Freier EF (1967) Determination of calcium in urine and serum by atomic absorption spectrophotometry (AAS). Clin Chem 13:101–114
Roos JTH, Price WJ (1971) Mechanism of interference and releasing action in atomic-absorption spectrometry. I. The effect of citric acid on iron. Spectrochimica Acta 26B:279–284
Yuh WTC, Fisher DS, Harms SE, Maravilla KR, Price AC, Runge VM (1992) Phase III multicenter trial of high-dose gadolinium MR imaging in the evaluation of brain metastases. Book of Abstracts, 30th Annual Meeting Am Soc Neuroradiol, p 76, paper 113
Marchal G, Michiels J, Bosmans H, van Hecke P (1992) Contrast-enhanced MRA of the brain. Comput Assit Tomogr 16: 25–19
Muehler A, Sreed M, Wendland MF, Brasch RC, Higgins CB (1991) Nonionic versus ionic MR contrast media: hemodynamic studies (abstr). Book of Abstracts, Soc Magn Reson Med, p 1185
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Demaerel, P., Marchal, G., Wilms, G. et al. Gadodiamide injection at 0.1 and 0.3 mmol/kg body weight: a phase III double-blind, parallel, randomised clinical investigation of known or suspected cenctral nervous system lesions at 1.5 T. Neuroradiology 36, 355–359 (1994). https://doi.org/10.1007/BF00612117
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00612117