Summary
-
1.
Intracellular recordings of intensity response functions (V/log I curves) were made at different adaptation states at different times during a 24th period, from single photoreceptors in the central region of the eye of the Australian crayfishCherax destructor (Clark 1936).
-
2.
The intensity that elicits a criterion response (50% of the maximum) is less at night than in the day in both the dark-adapted (DA) and lightadapted (LA) states.
-
3.
Within the first 30 s of light-adaptation there is an extremely fast initial decrease in sensitivity, while in the latter stages of dark-adaptation there is a slow but steady increase, neither of which can be correlated with the position of the screening pigments.
-
4.
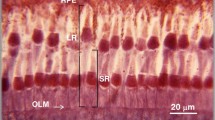
The time courses for the movement of screening-pigment on lightor dark-adaptation during both day and night were measured using light microscopy. As found by de Bruin and Crisp (1957) in several species of prawn and Frixione et al. (1979) in the crayfishProcambarus, the proximal pigment migrates faster than the distal pigment on light-adaptation. On dark-adaptation, the distal pigment migrates faster than the proximal pigment, the latter only carrying out the first stage of dark-adaptation during the day.
-
5.
It is concluded that there are three variable systems affecting sensitivity in a reflecting superposition eye: (a) The effective rhabdom cross-sectional area as determined by the position of proximal pigment; (b) the blur circle diameter and intensity determined by the position of the distal pigments and the width of the clear zone, and (c) transduction gain.
Similar content being viewed by others
Abbreviations
- LA :
-
light-adapted
- DA :
-
dark-adapted
- PPI :
-
Proximal pigment index
- DPI :
-
Distal pigment index
References
Aréchiga H, Fuentes B (1970) Correlative changes between retinal shielding pigment position and electroretinogram in crayfish. Physiologist 13:137
Aréchiga H, Fuentes B, Barrera B (1973) Circadian rhythm of responsiveness in crayfish visual units. In: Salanki J (ed) Neurobiology of invertebrates. Hungarian Academy of Sciences, Budapest, pp 403–421
Aréchiga H, Fuentes-Pardo B, Barrera-Mera B (1974) Influence of retinal shielding pigments on light sensitivity in the crayfish. Acta Physiol Lat Am 24:601–611
Aréchiga H, Wiersma CAG (1969) Circadian rhythm of responsiveness in crayfish visual units. J Neurobiol 1:71–85
Barrera-Mera B (1976) Effects of cerebroid ganglion lesions on ERG circadian rhythm of the crayfish. Physiol Behav 17:59–64
Bruin GHP de, Crisp DJ (1957) The influence of pigment migration on vision of higher Crustacea. J Exp Biol 34:447–463
Bryceson KP, McIntyre PD (1983) Image quality and acceptance angle in a reflecting superposition eye. J Comp Physiol 151:367–380
Doujak FE (1984) Electrophysiological measurement of photoreceptor membrane dichroism and polarization sensitivity in a grapsid crab. J Comp Physiol A 154:597–605
Eguchi E (1965) Rhabdom structure and receptor potentials in single crayfish retinular cells. J Cell Comp Physiol 66:411–430
Frixione E, Aréchiga H, Tsutsumi V (1979) Photomechanical migrations of pigment granules in insect superposition eyes. J Neurobiol 10:573–590
Fuortes MGF, Hodgkin AL (1964) Changes in time scale and sensitivity in the ommatidia ofLimulus. J Physiol 172:239–263
Fuortes MGF, Yeandle S (1964) Probability of occurrence of discrete potential waves in the eye ofLimulus. J Gen Physiol 47:443–463
Glantz RM (1968) Light adaptation in the photoreceptor of the crayfish,Procambarus clarki. Vision Res 8:1407–1421
Glantz RM (1972) Visual adaptation: a case of non-linear summation. Vision Res 12:103–109
Höglund G (1966) Pigment migration, light screening and receptor sensitivity in the compound eye of nocturnal Lepidoptera. Acta Physiol Scand 69:5–56
Horridge GA, Mimura K, Hardie RC (1976) Fly photoreceptors III. Angular sensitivity as a function of wavelength and the limits of resolution. Proc R Soc Lond B 194:151–177
Kaplan E, Barlow RB (1980) Circadian clock inLimulus brain increases response and decreases noise of retinal photoreceptors. Nature 286:393–395
Nirschfeld K (1974) The absolute sensitivity of lens and compound eyes. Z Naturforsch 29c: 592–595
Land MF (1981) Optics and vision in invertebrates. In: Autrum H (ed) Vision in invertebrates. Handbook of sensory physiology, vol VII/6B. Springer, Berlin Heidelberg New York, pp 471–592
Larimer JL, Smith JTF (1980) Circadian rhythm of retinal sensitivity in crayfish: Modulation by the cerebral and optic ganglia. J Comp Physiol 136:313–326
Laughlin SB, Hardie RC (1978) Common strategies for light adaptation in the peripheral visual systems of fly and dragonfly. J Comp Physiol 128:319–340
Lilly-white PG (1977) Single photon signals and transduction in an insect eye. J Comp Physiol 122:189–200
Matić T, Laughlin SB (1981) Changes in intensity-response function of an insect's photoreceptors due to light adaptation. J Comp Physiol 145:169–177
Olivo RF, Larsen ME (1978) Brief exposure to light initiates screening pigment migration in retinula cells of the crayfishProcambarus. J Comp Physiol 125:91–96
Page TL, Larimer JL (1975) Neural control of circadian rhythmicity in the crayfis. II. The ERG amplitude rhythm. J Comp Physiol 97:81–96
Sanchez JA, Fuentes-Pardo B (1977) Circadian rhythm in the amplitude of the electroretinogram in the isolated eyestalk of the crayfish. Comp Biochem Physiol 56A:601–605
Scholes JH (1965) Discrete sub-threshold potentials from the dimly lit insect eye. Nature 202:572–573
Snyder A (1979) The physics of vision in compound eyes. In: Autrum H (ed) Vision in invertebrates. Handbook of sensory physiology, vol VII/6 A. Springer, Berlin Heidelberg New York, pp 225–313
Vogt K (1975) Zur Optik des Flußkrebsauges. Z Naturforsch 30c:691
Walcott B (1971) Unit studies on the receptor movement in the retina ofLethocerus (Belostomatidae, Hemiptera). Z Vergl Physiol 74:17–25
Walcott B (1974) Unit studies on light-adaptation in the retina of the crayfishCherax destructor. J Comp Physiol 94:207–218
Yeandle TC (1958) Evidence of quantized slow potentials in the eye ofLimulus. Am J Ophthalmol 46:82–86
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bryceson, K.P. Diurnal changes in photoreceptor sensitivity in a reflecting superposition eye. J. Comp. Physiol. 158, 573–582 (1986). https://doi.org/10.1007/BF00603801
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00603801