Summary
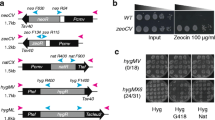
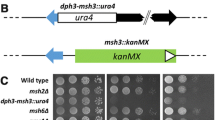
A plasmid construct carrying the hygromycin phosphotransferase (hph) gene fused to the expression elements of the trpC gene of Aspergillus nidulans was used to obtain hygromycin B (Hyg)-resistant transformants of Neurospora crassa. The plasmid does not have any homology with the N. crassa genome. Here we demonstrate that most of the transformants arise from integration of the transforming DNA into only one of the nuclei present in the protoplasts. Furthermore, in most of the transformants the integrated transforming DNA is physically stable after growth of the transformants for about 25 nuclear divisions without Hyg selection, in spite of being present in multiple copies. In transformants carrying only a single insertion, phenotypic expression of the hph gene remains unaltered in conidial isolates obtained withoug Hyg selection. On the other hand, about 40% of transformants harbouring plasmid DNA integrated at more than one location yield conidial isolates showing reversible inactivation of the hph genes. Interestingly, the presence of methylated cytosine residues in the integrated DNA is strongly correlated with the number of plasmid copies. The hph genes are heavily methylated in transformants harbouring multiple copies but not in those harbouring only one copy of the plasmid. Phenotypic expression of the inactive hph genes can be restored by growing the transformants either under Hyg selection pressure or in the presence of 5-azacytidine. In the first case the hph genes are again inactivated when Hyg selection pressure is removed, while the activation of the hph gene by 5-azacytidine gives stable Hygr strains.
Similar content being viewed by others
References
Antequera F, Boyes J, Bird A (1990) High levels of de novo methylation and altered chromatin structure at CpG islands in cell lines. Cell 62:503–514
Bull JH, Wootton JC (1984) Heavily methylated amplified DNA in transformants of Neurospora crassa. Nature 310:701–704
Davis RH, deSerres FJ (1970) Genetic and microbiological research techniques for Neurospora crassa. Methods Enzymol 17A:79–143
Doerfler W (1991) Patterns of DNA methylation — evolutionary vestiges of foreign DNA inactivation as a host defense mechanism. A proposal. Biol Chem Hoppe Seyler 372:557–564
Faugeron G, Rhounim L, Rossignol J (1990) How does the cell count the number of ectopic copies of a gene in the prcmeiotic inactivation process acting in Ascobolus immersus? Genetics 124:585–591
Fincham JRS (1989) Transformation in fungi. Microbiol Rev 53:148–170
Gritz L, Davies J (1983) Plasmid-encoded hygromycin B resistance: the sequence of hygromycin B phosphotransferase gene and its expression in Escherichia coli and Saccharomyces cerevisiae. Gene 25:179–188
Hanish J, McClelland M (1988) Activity of DNA modification and restriction enzymes KGB, a potassium glutamate buffer. Gene Anal Techn 15:105–107
Kuehn MR, Stoye JP (1992) Insertional mutagenesis and mouse development. In: Russo VEA, Brody S, Cove DJ, Ottolenghi S (eds) Development — the molecular genetic approach. Springer Verlag, Heidelberg pp 420–439
Lee SB, Milgroom MG, Taylor JW (1988) A rapid, high yield mini-prep method for isolation of total genomic DNA from fungi. Fungal Genet Newslett 35:23–24
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: A laboratory manual. Cold Spring Harbor Laboratory Press, New York
Matzke MA, Primig M, Trnovsky J, Matzke AJM (1989) Reversible methylation and inactivation of marker genes in sequentially transformed tobacco plants. EMBO J 8:643–649
Mishra NC (1979) DNA-mediated genetic changes in Neurospora crassa. J Gen Microbiol 113:255–259
Nelson M, McClelland M (1991) Site-specific methylation: effect on DNA modification methyltransferases and restriction endonucleases. Nucleic Acids Res 19:2045–2071
Orbach MJ, Schneider WP, Yanofsky C (1988) Cloning of methylated transforming DNA from Neurospora crassa in Escherichia coli. Mol Cell Biol 8:2211–2213
Palmiter RD, Brinster RL (1986) Germ-line transformation of mice. Annu Rev Genet 20:465–499
Razin A, Cedar H (1991) DNA methylation and gene expression. Microbiol Rev 55:451–458
Rothstein R (1991) Targeting, disruption, replacement, and allele rescue: integrative DNA transformation in yeast. Methods Enzymol 194:281–301
Russell PJ, Rodland KD, Rachlin EM, McCloskey JA (1987) Differential DNA methylation during the vegetative life cycle of Neurospora crassa. J Bacteriol 169:2902–2905
Scheid OM, Paszkowski J, Potrykus I (1991) Reversible inactivation of a transgene in Arabidopsis thaliana. Mol Gen Genet 228:104–112
Selker EU (1990A) Premeiotic instability of repeated sequences in Neurospora crassa. Annu Rev Genet 24:579–613
Selker EU (1990B) DNA methylation and chromatin structure: a view from below. TIBS 15:103–107
Selker EU, Stevens JN (1985) DNA methylation at asymmetric sites is associated with numerous transition mutations. Proc Natl Acad Sci USA 82:8114–8118
Selker EU, Jensen C, Richardson GA (1987) A portable signal causing faithful DNA methylation de novo in Neurospora crassa. Science 238:48–53
Staben C, Jensen B, Singer M, Pollock J, Schechtman M, Kinsey J, Selker E (1989) Use of a bacterial hygromycin B resistance gene as a dominant selectable marker in Neurospora crassa transformation. Fungal Genet Newslet 36:79–81
Vollmer SJ, Yanofsky C (1986) Efficient cloning of genes of Neurospora crassa Proc Natl Acad Sci USA 83:4869–4873
Weih F, Nitsch D, Reik A, Schütz G, Becker PB (1991) Analysis of CpG methylation and genomic footprinting at the tyrosine aminotransferase gene: DNA methylation alone is not sufficient to prevent protein binding in vivo. EMBO J 10:2559–2567
Weising K, Schell J, Kahl G (1988) Foreign genes in plants: transfer, structure, expression, and applications. Annu Rev Genet 22:421–477
Author information
Authors and Affiliations
Additional information
Dedicated to Dr. T.A. Trautner on the occasion of his 60th birthday
Rights and permissions
About this article
Cite this article
Pandit, N.N., Russo, V.E.A. Reversible inactivation of a foreign gene, hph, during the asexual cycle in Neurospora crassa transformants. Molec. Gen. Genet. 234, 412–422 (1992). https://doi.org/10.1007/BF00538700
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00538700