Abstract
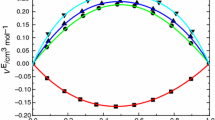
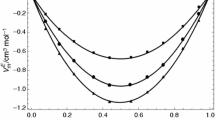
Viscosity coefficients for binary mixtures of hexafluorobenzene with benzene, toluene, para-xylene, and mesitylene have been measured along the saturation line at temperatures from 15 to 120°C using specially designed capillary viscometers. Densities were measured using a pyknometer and volume-change apparatus. Deviations of the viscosities from a rectilinear dependence on mole fraction are consistent with enhanced interactions between unlike species, which increase with increasing number of methyl groups on the aromatic hydrocarbon and decrease with increasing temperature. The application of the Grunberg and Nissan equation, the Hildebrand equation, and energy of activation theories to these results is examined.
Similar content being viewed by others
References
C. R. Patrick and G. S. Prosser, Nature 187:1021 (1960).
W. A. Duncan and F. L. Swinton, Trans. Faraday Soc. 62:1082 (1966).
E. McLaughlin and C. E. Messer, J. Chem. Soc. A 1106 (1966).
J. C. A. Boeyens and F. H. Herbstein, J. Phys. Chem. 69:2153 (1965).
T. Dahl, Acta Chem. Scand. Ser. A A29:699 (1975).
T. Dahl, Acta Chem. Scand. 27:995 (1973).
D. F. R. Gilson and C. A. McDowell, Can. J. Chem. 44:945 (1966).
T. Dahl, Acta Chem. Scand. 26:1569 (1972).
A. D. Bedsole and Z. L. Taylor, Jr., J. Ala. Acad. Sci. 39:270 (1968).
A. K. M. Masood, A. M. North, R. A. Pethrick, M. Towland, and F. L. Swinton, J. Chem. Thermodyn. 9:133 (1977),
D. V. Fenby, I. A. McLure, and R. L. Scott, J. Phys. Chem. 70:6023 (1966).
J. Vrbancich and G. L. D. Ritchie, J. Chem. Soc. Faraday II 76:648 (1980).
A. K. M. Masood, A. M. North, R. A. Pethrick, M. Towland, and F. L. Swinton, Adv. Mol. Relax. Process. 9:153 (1976).
D. A. Bauer, J. I. Brauman, and R. Pecora, J. Chem. Phys. 63:53 (1975).
J. H. Dymond, J. Robertson, and J. D. Isdale, Int. J. Thermophys. 2:223 (1981).
J. H. Dymond, N. Glen, J. Robertson, and J. D. Isdale, J. Chem. Thermodyn. 14:1149 (1982).
J. H. Dymond and K. J. Young, Int. J. Thermophys. 1:331 (1980).
G. D. Wedlake, J. H. Vera, and G. A. Ratcliff, Rev. Sci. Instrum. 50:93 (1979).
Handbook of Chemistry and Physics, 61st ed. (C.R.C. Press, Boca Raton, Fla., 1980–1981).
Selected Values of Properties of Hydrocarbons and Related Compounds, American Petroleum Research Project 44 (Thermodynamic Research Centre, Texas A&M University, College Station, 1948, 1949, 1972).
A. Weissberger, Techniques of Organic Chemistry I. Physical Methods, Vol.I (Interscience, New York, 1959), pp. 85–86.
W. A. Duncan, J. P. Sheridan, and F. L. Swinton, Trans. Faraday Soc. 62:1090 (1966).
J. L. Hales and R. Townsend, J. Chem. Thermodyn. 6:111 (1974).
R. Meyer, A. Barlatier, and J. Metzger, J. Chim. Phys. Physiochim. Biol. 68:417 (1971).
S. S. Chen and B. J. Zwolinski, J. Chem. Thermodyn. 7:251 (1975).
J. L. Hales and R. Townsend, J. Chem. Thermodyn. 4:763 (1972).
F. Kimura and S. Marakami, Fluid Phase Equil. 3:93 (1979).
J. Timmermans, Physico-Chemical Constants of Pure Organic Compounds. Vol. II (Elsevier, Amsterdam, London, New York, 1965).
H. L. Clever and K.-Y. Hsu, J. Chem. Thermodyn. 7:435 (1975).
J. A. Riddick and W. B. Burger, Organic Solvents, Techniques of Chemistry, Vol. II, A. Weissberger, ed. (Wiley Interscience, New York, 1970).
H. L. Clever and K.-Y. Hsu, J. Chem. Thermodyn. 10:213 (1978).
W. Woycicki and K. W. Sadowska, Bull. Acad. Pol. Sci. Ser. Sci. Chim. 16:531 (1968).
Int. Data Ser., Selec. Data Mix. Ser. A 203 (1974).
A. Nissema and A. Kuvaja, Suomen Kemistilehti B 45:206 (1972).
M. J. Mussche and L. A. Verhoeye, J. Chem. Eng. Data 20:46 (1975).
H. M. N. H. Irving and R. B. Simpson, J. Inorg. Nucl. Chem. 34:2241 (1972).
M. S. Medani and M. A. Hasan, Can. J. Chem. Eng. 55:203 (1977).
J. Timmermans, Physico-Chemical Constants of Pure Organic Compounds (Elsevier, New York, Amsterdam, London, Brussels, 1950).
A. Nissema and P. Kokkonen, Finn. Chem. Lett. 1:7 (1979).
P. Kokkonen and A. Nissema, Finn. Chem. Lett. 3:69 (1979).
S. Ruenkrairergsa, D. V. Fenby, and D. E. Jones, J. Chem. Thermodyn. 5:347 (1973).
S. Glasstone, K. J. Laidler, and H. Eyring, Theory of Rate Processes (McGraw-Hill, New York, 1941), Chap. 9.
J. B. Irving, N.E.L. Report No. 631 (National Engineering Laboratory, East Kilbride, Glasgow, 1977).
L. Grunberg and A. H. Nissan, Nature (Lond.) 164:799 (1949).
C. L. Watkins and W. S. Brey, Jr., J. Phys. Chem. 74:235 (1970).
A. J. Batschinski, Z. Physik. Chem. 84:643 (1913).
J. H. Hildebrand, Science 174:490 (1971).
E. Ertl and F. A. L. Dullien, J. Phys. Chem. 77:3007 (1973).
G. L. Bertrand, Ind. Eng. Chem. Fundam. 16:492 (1977).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dymond, J.H., Robertson, J. Transport properties of nonelectrolyte liquid mixtures—VI. Viscosimetric study of binary mixtures of hexafluorobenzene with aromatic hydrocarbons. Int J Thermophys 6, 21–41 (1985). https://doi.org/10.1007/BF00505790
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00505790