Abstract
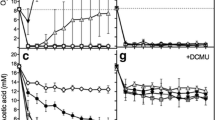
In cells of the green alga Chlorella fusca, which contain active hydrogenase(s), the concentration of ATP, NADH and NADPH were measured during a 5 h period of anaerobiosis in the dark and upon subsequent illumination with high light intensities (770 W/m2), conditions which favour optimal hydrogen photoproduction.
ATP concentrations were also determined in cells of Chlorella fusca, whose hydrogenase was inactivated prior to illumination, and in cells of Chlorella vulgaris which do not contain hydrogenase. In the dark, the ATP concentration increased slightly during anaerobiosis in cells with active hydrogenase. This increase in ATP concentration was accompanied by an increase of NADH and a decrease of NADPH content.
Upon illumination, the ATP content increased in cells with an active hydrogenase, whereas the NADH content decreased. The rate of phosphorylation was twice that observed in cells without active hydrogenase.
This ATP synthesis in the light was not inhibited by 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) (10 μmol/l) nor by carbonylcyanide-3-chlorophenyl-hydrazone (CCCP) (1 μmol/l) but was diminished by 500 μmol/l dibromothymoquinone (DBMIB) and 6 μmol/l carbonylcyanide-3-chlorophenyl-hydrazone (CCCP).
It was concluded that an active hydrogenase can support ATP production under anaerobic conditions in the dark as well as in the light. NADH might serve in vivo as electron donor for a fermentative production of hydrogen in the light.
Possible mechanisms underlying ATP production under anaerobiosis and hydrogen productive conditions are discussed.
Similar content being viewed by others
Abbreviations
- CCCP:
-
Carbonylcyanide-3-chlorophenyl-hydrazone
- DBMIB:
-
dibromothymoquinone
- DCMU:
-
3-(3,4-dichlorophenyl)-1,1-dimethylurea
- FCCP:
-
carbonylcyanide-p-trifluormethoxyphenyl-hydrazone
- HEPES:
-
N-2-hydroxyethylpiperazin-N′-2-ethan-sulfonic acid
- PSI:
-
II, photosystem I, II respectively
- PQ:
-
plastoquinone
References
Adams MWW, Mortenson LE, Chen JS (1981) Hydrogenase. Biochim Biophys Acta 594:105–176
Ben-Amotz A, Gibbs M (1975) H2 metabolism in photosynthetic organisms. II. Light dependent H2 evolution by preparations from Chlamydomonas, Scenedesmus and Spinach. BBRC 64:355–359
Bishop NI, Frick M, Jones LW (1977) Photohydrogenproduction in green algae: water serves as the primary substrate for hydrogen and oxygen production. In: Mitsui A, Miyachi S, San Pietro A, Tamura S (eds) Biological solar energy conversion. Academic Press, New York, pp 23–51
Gfeller RP, Gibbs M (1984) Fermentative metabolism of Chlamydomonas reinhardii. I. Analysis of fermentative products from starch in dark and light. Plant Physiol 75:212–218
Godde D (1982) Evidence for a membrane bound NADH-PQ-oxidoreductase in Chlamydomonas reinhardii CW-15. Arch Microbiol 131:197–202
Godde D, Trebst A (1980) NADH as electron donor for the photosynthetic membrane of Chlamydomonas reinhardii. Arch Microbiol 127:245–252
Greenbaum E (1980) Simultaneous photoproduction of hydrogen and oxygen by photosynthesis. In: Scott CD (ed) Biotechnology and bioengineering Symposium No 10. Second Symposium on Biotechnology in energy production and conservation. Wiley and Sons, New York, pp 1–14
Greenbaum E, Guillard RRL, Sunda WG (1983) Hydrogen and oxygen photoproduction by marine algae. Photochem Photobiol 37:649–655
Grimme LH, Boardman NK (1972) Photochemical activities of a particle fraction P1 derived from the green algae Chlorella, Biochem Biophys Res Comm 49:1617–1623
Hallenbeck PC, Benemann JR (1979) Hydrogen from algae. In: Barber J (ed) Photosynthesis in relation to model systems. Elsevier, Amsterdam, pp 331–364
Healey FP (1970) The mechanism of H2 evolution by Chlamydomonas moewousii. Plant Physiol 45:153–159
Kessler E (1973) Effect of anaerobiosis on photosynthetic reactions and nitrogen metabolism of algae with and without hydrogenase. Arch Mikrobiol 93:91–100
Kessler E (1974) Hydrogenase, photoreduction and anaerobic growth. In: Stewart WDP (ed) Algal physiology and biochemistry. Blackwell, Oxford, pp 456–473
Klein U, Betz A (1978) Fermentative metabolism of hydrogenevolving Chlamydomonas moewousii. Plant Physiol 61:953–956
Kreuzberg K (1981) Algal fermentation, a promising step in biomass conversation. In: Comm Eur Communities (REP) EUR 7091, 1st Energy Biomass Conference, pp 709–714
Kreuzberg K (1984) Starch fermentation via a formate producing pathway in Chlamydomonas reinhardii, Chlorogonium elongatum and Chlorella fusca. Plant Physiol 61:87–94
Lovgren T, Peacock R, Lavi J, Karp M, Raunio R (1982) The bioluminescent assay of NADH and NADPH. Intern Laboratory, July/Aug., pp 58–67
Mahro B (1983) Die Wasserstoff (H2)-Photoproduktion der Grünalgae Chlorella fusca: Endogene und exogene Voraussetzung für ihre Optimierung. Diss Univ Bremen
Mahro B, Grimme LH (1982) H2 Photoproduction by green algae: The significance of anaerobic pre-incubation periods and of high light intensities for H2-photoproductivity of Chorella fusca. Arch Microbiol 132:82–86
Mahro B, Grimme LH (1986) Improving the photosynthetic H2-productivity of the green alga Chlorella fusca by physiologically directed O2 avoidance and ammonium stimulation. Arch Microbiol 144:25–28
Persanov VM, Gogotov IN (1978) Hydrogenase activity of Chlorella vulgaris cells. Microbiology 47:173–177
Stuart TS, Kaltwasser H (1970) Photoproduction of hydrogen by photosystem I of Scenedesmus. Planta 91:302–313
Vinayakumar M, Kessler E (1975) Physiological and biochemical contribution to the taxonomy of the genus Chlorella. X. Products of glucose fermentation. Arch Microbiol 103:13–19
Weaver PF, Lien S, Seibert M (1980) Photobiological production of hydrogen. Solar energy 24:3–45
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mahro, B., Küsel, A.C. & Grimme, L.H. The significance of hydrogenase activity for the energy metabolism of green algae: anaerobiosis favours ATP synthesis in cells of Chlorella with active hydrogenase. Arch. Microbiol. 144, 91–95 (1986). https://doi.org/10.1007/BF00454962
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00454962