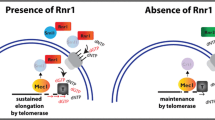
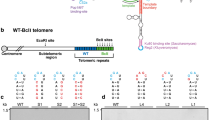
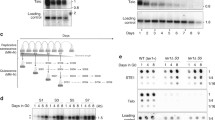
Summary
Natural termini from macronuclear DNA of the ciliated protozoans Tetrahymena thermophila and Oxytricha fallax can support telomere formation in yeast. However, plasmids carrying these ciliate termini are modified by the addition of DNA which hybridizes to the synthetic oligonucleotide poly [d(C-A)], a sequence which also hybridizes to terminal restriction fragments from yeast chromosomes but not to Tetrahymena or Oxytricha macronuclear DNAs. Thus, in yeast, the creation of new telomeres on ciliate termini involves the acquisition of yeast-specific terminal sequences presumably by either recombination or non-templated DNA synthesis. The RAD52 gene is required for the majority of yeast mitotic and meiotic recombination events. Moreover, the absence of an active RAD52 gene product results in high rates of chromosome loss. Here we demonstrate that terminal restriction fragments from Tetrahymena macronuclear ribosomal DNA (rDNA) support the formation of modified telomeres in a yeast strain carrying a defect in the RAD52 gene. Moreover, linear plasmids bearing these modified ciliate termini are stably propagated in rad52 − cells.
Similar content being viewed by others
References
Bergold PJ, Campbell GR, Littau VC, Johnson EM (1983) Cell 32:1287–1299
Blackburn EH, Gall JG (1978) J Mol Biol 120:33–53
Boswell RE, Klobutcher LA, Prescott DM (1982) Proc Natl Acad Sci USA 79:3255–3259
Dani GM, Zakian VA (1983) Proc Natl Acad Sci USA 80:3406–3410
Davis RW, Thomas M, Cameron J, St John TP, Scherer S, Padget RA (1980) Methods Enzymol 65:404–411
Dunn B, Szauter P, Pardue ML, Szostak JW (1984) Cell 39:191–201
Game JC, Zamb TJ, Braun RJ, Resnick M, Roth RM (1980) Genetics 94:51–68
Heumann JM (1976) Nucleic Acids Res 3:3167–3171
Ho KSY (1975) Mutat Res 30:327–334
Holmquist GP, Dancis B (1979) Proc Natl Acad Sci USA 76:4566–4570
Jackson JA, Fink GR (1981) Nature 292:306–311
King BO, Yao MC (1982) Cell 31:177–182
Kiss GB, Amin AA, Pearlman RE (1981) Mol Cell Biol 1:535–543
Klobutcher LA, Swanton MT, Donini P, Prescott DM (1981) Proc Natl Acad Sci USA 78:3015–3019
Lawn RM, Heumann JM, Herrick G, Prescott DM (1978) Cold Spring Harbor Symp Quant Biol 42:483–492
Malone RE, Esposito RE (1980) Proc Natl Acad Sci USA 77:503–507
Mortimer RK, Contopoulou R, Schild D (1981) Proc Natl Acad Sci USA 78:5778–5782
Oka Y, Honjo T (1983) Nucleic Acids Res 11:4325–4333
Oka Y, Shiota S, Nakai S, Nishida Y, Okubo S (1980) Gene 10:301–306
Pluta AF, Dani GM, Spear BB, Zakian VA (1984) Proc Natl Acad Sci USA 81:1475–1479
Pluta AF, Kaine BP, Spear BB (1982) Nucleic Acids Res 10:8145–8154
Prakash S, Prakash L, Burke W, Montelone BA (1980) Genetics 94:31–50
Preer JR Jr, Preer LB (1979) J Protozool 26:14–18
Resnick MA, Martin P (1976) Mol Gen Genet 143:119–129
Shampay J, Szostak JW, Blackburn EH (1984) Nature 310:154–157
Stinchcomb DT, Mann C, Davis RW (1982) J Mol Biol 158:157–179
Szostak JW, Blackburn EH (1982) Cell 29:245–255
Walmsley RM, Szostak JW, Petes TD (1983) Nature 302:84–86
Walmsley RW, Chan CSM, Tye B-K, Petes TD (1984) Nature 310:157–160
Weiffenbach B, Haber JE (1981) Mol Cell Biol 1:522–534
Wild MA, Gall JG (1979) Cell 16:565–573
Zakian VA, Scott JF (1982) Mol Cell Biol 2:221–232
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zakian, V.A., Blanton, H.M. & Dani, G.M. Formation and stability of linear plasmids in a recombination deficient strain of yeast. Curr Genet 9, 441–445 (1985). https://doi.org/10.1007/BF00434048
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00434048