Abstract
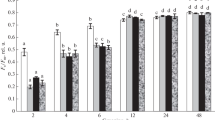
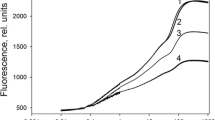
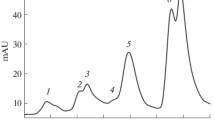
Mustard (Sinapis alba L.) seedlings were grown in the presence of herbicides (Difunon, Norflurazon) which inhibit carotenoid synthesis without affecting development, in darkness or in continuous far-red light. In strong white light (12,000 lx) the cotyledons of the herbicide-treated seedlings did not contain normal chloroplasts, but only small chlorophyll-free rudiments whose internal structure had almost disappeared. The plastid marker enzyme NADP-dependent glyceraldehyde-3-phosphate dehydrogenase was almost lacking. Plastid ribosomes and ribosomal RNAs were no longer detectable nor could synthesis of mature plastidal ribosomal RNAs be detected. Cytosolic ribosomes and rRNAs were not affected. Plastid DNA was apparently still intact as shown by restriction analysis. The appearance of marker enzymes of glyoxisomes, mitochondria and cytosol was not impaired while the level of marker enzymes of peroxisomes was drastically lowered. Accumulation of anthocyanin in mustard cotyledons was normal after a short, transient delay. Levels of representative enzymes of flavonoid biogenesis (phenylalanine ammonia-lyase, chalcone synthase) were somewhat increased rather than inhibited in the cotyledons of herbicide-treated, white-light-grown seedlings. The growth rate of hypocotyl and cotyledons was inhibited to the same extent in the herbicide-treated, white-light-grown seedling, although light inhibits growth of hypocotyls and promotes growth of cotyledons. Analysis of the data shows that photomorphogenesis of a herbicide-treated, white-light-grown seedling is normal, and is thus independent of plastid gene expression However, a ‘factor’ which coacts multiplicatively with phytochrome in determining the growth rate of the organs seems to originate from the plastids. Biogenesis of anthocyanin and synthesis of major enzymes of the flavonoid pathway are not affected adversely by a photooxidative elimination of plastid gene expression.
Similar content being viewed by others
Abbreviations
- cFR:
-
continuous far-red light
- cWL:
-
continuous white light
- DF:
-
Difunon
- GPD:
-
glyceraldehyde-3-phosphate dehydrogenase (GPD-NADP+, EC. 1.2.1.13; GPD-NAD+, EC 1.2.1.12)
- NF:
-
Norflurazon
- ptDNA:
-
plastid DNA
References
Bajracharya, D., Schopfer, P. (1979) Effect of light on the development of glyoxysomal functions in the cotyledons of mustard (Sinapis alba L.) seedlings. Planta 145, 181–186
Blume, D.E., McClure, J.W. (1980) Developmental effects of sandoz 6706 on activities of enzymes of phenolic and general metabolism in barley shoots grown in the dark or under low or high intensity light. Plant Physiol. 65, 238–244
Brünning, K.H., Drumm, H., Mohr, H. (1975) On the role of phytochrome in controlling enzyme levels in plastids. Biochem. Physiol. Pflanz. 168, 144–156
Cerff, R. (1973) Glyceraldehyde-3-phosphate dehydrogenases and glyoxylate reductase. I. Their regulation under continuous red and far-red light in the cotyledons of Sinapis alba L. Plant Physiol. 51, 76–81
Cerff, R., Kloppstech, K. (1982) Structural diversity and differential light control of mRNAs coding for angiosperm glyceraldehyde-3-phosphate dehydrogenases. Proc. Natl. Acad. Sci. USA 79, 7624–7628
Charriere-Ladreix, Y., Douce, R., Joyard, J. (1981) Characterization of 0-methyltransferase activities associated with spinach chloroplast fractions. FEBS Lett. 133, 55–58
de Looze, S.M., Wagner, E. (1983) In vitro and in vivo regulation of chloroplast glyceraldehyde-3-phosphate dehydrogenase isozymes from Chenopodium rubrum. I. Purification and properties of isozymes. Physiol. Plant. 57, 231–237
Dubbelman, T.M.A.R., Van Steveninck, A.L., Van Steveninck, J. (1982) Hematoporphyrin-induced photooxidation and photodynamic cross-linking of nucleic acids and their constituents. Biochim. Biophys. Acta 719, 47–52
Ebel, J., Hahlbrock, K. (1982) Biosynthesis. In: The flavonoids: advances in research, pp. 641–679, Harborne, J.B., Marby, T.J., eds. Chapman and Hall, London New York
Ellis, R.J. (1981) Chloroplast proteins: synthesis, transport, and assembly. Annu. Rev. Plant Physiol. 32, 111–137
Feierabend, J., Kemmerich, P. (1983) Mode of interference of chlorosis-inducing herbicides with peroxisomal enzyme activities. Physiol. Plant. 57, 346–351
Feierabend, J., Schubert, B. (1978) Comparative investigation of the action of several chlorosis-inducing herbicides on the biogenesis of chloroplasts and leaf microbodies. Plant Physiol. 61, 1017–1022
Feierabend, J., winkelhüsener, T. (1982) Nature of photooxidative events in leaves treated with chlorosis-inducing herbicides. Plant Physiol. 70, 1277–1282
Frosch, S., Bergfeld, R., Mohr, H. (1976) Light control of plastogenesis and ribulosebisphosphate carboxylase levels in mustard seedling cotyledons. Planta 133, 53–56
Frosch, S., Jabben, M., Bergfeld, R., Kleinig, H., Mohr, H. (1979) Inhibition of carotenoid biogenesis by the herbicide SAN 9789 and its consequences for the action of phytochrome on plastogenesis. Planta 145, 497–505
Frosch, S., Mohr, H. (1980) Analysis of ligh-controlled accumulation of carotenoids in mustard (Sinapis alba L.) seedlings. Planta 148, 279–286
Gupta, S., Acton, G.J. (1979) Purification to homogenity and some properties of L-phenylalanine ammonia-lyase of irradiated mustard (sinapis alba L.) cotyledons. Biochim. Biophys. Acta 570, 187–197
Hock, B. (1973) Isoenzyme der Malat-Dehydrogenase aus Wassermelonen Keimlingen: Mikroheterogenität und deren Aufhebung bei der Samenkeimung. Planta 110, 329–344
Hock, B., Mohr, H. (1965) Eine quantitative Analyse von Wachstumsvorgängen im Zusammenhang mit der Photomorphogenese von Senfkeimlingen (Sinapis alba L.). Planta 65, 1–16
Kalthofen, H. (1981) Koaktionsanalyse — eine Methode zur Analyse des Zusammenwirkens zweier Einflußgrößen. Biol. Rundsch. 19, 155–173
Kasemir, H., Mohr, H. (1982) Coaction of three factors controlling chlorophyll and anthocyanin synthesis. Planta 156, 282–288
Kochar, V.M., Kochar, S., Mohr, H. (1981) An analysis of the action of light on betalain synthesis in the seedlings of Amaranthus caudatus, var. viridis. Planta 151, 81–87
Knogge, W., Beulen, C., Weisenboeck, G. (1981) Distribution of phenylalanine ammonia-lyase and 4-coumarate: CoA ligase in oat primary leaf tissue. Z. Naturforsch. Teil C 36, 389–395
Lange, H., Shropshire, W., Mohr, H. (1971) An analysis of phytochrome-mediated anthocyanin synthesis. Plant Physiol. 47, 649–655
Link, G. (1981) Cloning and mapping of the chloroplast DNA sequences for two messenger RNAs from mustard (Sinapis alba L.). Nucleic Acids Res. 9, 3681–3694
Link, G., Chambers, S.E., Thompson, J.A., Falk, H. (1981) Size and physical organization of chloroplast DNA from mustard (Sinapis alba L.). Mol. Gen. Genet. 181, 454–457
Link, G., Coen, D.M., Bogorad, L. (1978) Differential expression of the gene for the large subunit of ribulosebisphosphate carboxylase in maize leaf cell types. Cell 15, 725–731
Mohr, H. (1959) Der Lichteinfluß auf das Wachstum der Keimblätter bei Sinapis alba L. Planta 53, 219–245
Mohr, H. (1966) Untersuchungen zur phytochrominduzierten Photomorphogenese des Senfkeimlings (Sinapis alba L.). Z. Pflanzenphysiol. 54, 63–83
Mohr, H. (1972) Lectures on Photomorphogenesis. Springer, Berlin Heidelberg New York
Mohr, H. (1982) Phytochrome and gene expression. In: Trends in photobiology, pp. 515–530, Hélène, C., Charlier, M., Montenay-Garestier, Th., Laustriat, G., eds. Plenum Press, New York London
Nishizawa, A.N., Wolosiuk, R.A., Buchanan, B.B. (1979) Chloroplast phenylalanine ammonia-lyase from spinach leaves Planta 145, 7–12
Oelze-Karow, H., Rösch, H., Mohr, H. (1983) Prevention by phytochrome of photodelay in chlorophyll accumulation. Photochem. Photobiol. 37, 565–569
Ohlrogge, J.B. (1982) Fatty acid synthetase: plants and bacteria have similar organization. Trends Biochem. Sci. 7, 386–387
Podstolski, A. (1981) Chloroplast-released inhibitor of phenylalanine ammonia lyase from barley (Hordeum vulgare) seedlings. Physiol. Plant. 52, 407–410
Reiß, T. (1983) Wechselwirkungen zwischen Plastidenkompartiment und Cytoplasma bei der Photomorphogenese. Ph.D. thesis, University of Freiburg
Saunders, J.A., McClure, J.W. (1975) Phytochrome controlled phenylalanine ammonia lyase in Hordeum vulgare plastids. Phytochemistry 14, 1285–1289
Sautter, C., Hock, B. (1982) Fluorescence immunohistochemical localization of malat dehydrogenase isoenzymes in watermelon cotyledons. Plant Physiol. 70, 1162–1168
Schopfer, P. (1977) Phytochrome control of enzymes. Annu. Rev. Plant Physiol. 28, 223–252
Schopfer, P., Mohr, H. (1972) Phytochrome-mediated induction of phenylalanine ammonia-lyase in mustard seedlings. Plant Physiol. 49, 8–10
Southern, E.M. (1975) Detection of specific sequences among DNA fragments separated by gel electrophoresis. J. Mol. Biol. 98, 503–517
Stumpf, P.K. (1980) Biosynthesis of saturated and unsaturated fatty acids. In: The biochemistry of plants, vol. 4: Lipids: structure and function, pp. 177–204, Stumpf, P.K., Conn, E.E., eds. Academic Press, New York London
Thien, W., Schopfer, P. (1982) Control by phytochrome of cytoplasmic precursor rRNA synthesis in the cotyledons of mustard seedlings. Plant Physiol. 69, 1156–1160
Wagner, L., Bienger, J., Mohr, H. (1967) Die Steigerung der durch Phytochrom bewirkten Anthocyansynthese des Senfkeimlings (Sinapis alba L.) durch Chloramphenicol. Planta 75, 1–9
Weissenboeck, G., Plesser, A., Trinks, K. (1976) Flavonoidgehalt und Enzymaktivitäten isolierter Haferchloroplasten (Avena sativa L.) Ber. Dtsch. Bot. Ges. 89, 457–472
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Reiß, T., Bergfeld, R., Link, G. et al. Photooxidative destruction of chloroplasts and its consequences for cytosolic enzyme levels and plant development. Planta 159, 518–528 (1983). https://doi.org/10.1007/BF00409141
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00409141