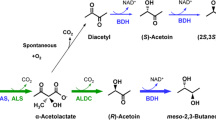
Abstract
Enterobacter aerogenes, Aeromonas hydrophila, Serratia marcescens and Staphylococcus aureus possessing L(+)-butanediol dehydrogenase produced mainly meso-butanediol and small amounts of optically active butanediol; Acetobacter suboxydans, Bacillus polymyxa and Erwinia carotovora containing D(-)-butanediol dehydrogenase produced more optically active butanediol than meso-butanediol. Resting and growing cells of these organisms oxidized only one enantiomer of racemic butanediol. The D(-)-butanediol dehydrogenase from Bacillus polymyxa was partially purified (30-fold) with a specific activity of 24.5. Except NAD and NADH no other cofactors were required. Optimum pH-values for oxidation and reduction were pH 9 and pH 7, respectively. The optimum temperature was about 60°C. The molecular weight was 100000 to 107000. The K m-values were 3.3 mM for D(-)-butanediol, 6.25 mM for meso-butanediol, 0.53 mM for acetoin, 0.2 mM for NAD, 0.1 mM for NADH, 87 mM for diacetyl, 38 mM for 1,2-propanediol; 2,3-pentanedion was not a substrate for this enzyme. The L(+)-butanediol dehydrogenase from Serratia marcescens was purified 57-fold (specific activity 22.3). Besides NAD or NADH no cofactors were required. The optimum value for oxidation was about pH 9 and for reduction pH 4.5. The optimum temperature was 32–36°C. The molecular weight was 100000 to 107000. The K m-values were 5 mM for meso-butanediol, 10 mM for racemic butanediol, 6.45 for acetoin, 1 mM for NAD, 0.25 mM for NADH, 2.08 mM for diacetyl, 16.7 mM for 2,3-pentanedion and 11.8 mM for 1,2-propanediol.
Similar content being viewed by others
Abbreviations
- Bud:
-
2,3-butanediol
- DH:
-
dehydrogenase
References
Aubert, J. P., Gavard, R.: Étude de la 2,3 butanediol-déshydrogenase avec un extract enzymatique bactérien. Compte rendus Acad. Sci. (Paris) 233, 1320–1322 (1951)
Bryn, K., Hetland, Ø., Størmer, F. C.: The reduction of diacetyl and acetoin in Aerobacter aerogenes—Evidence for one enzyme catalyzing both reactions. Eur. J. Biochem. 18, 116–119 (1971)
Guymon, J. F., Crowell, E. A.: Direct gas chromatographic determination of levo- and meso-2,3-butanediols in wines and factors affecting their formation. Am. J. Enol. Vitic. 18, 200–209 (1967)
Hetland, Ø., Olsen, B. R., Christensen, T. B., Størmer, F. C.: Diacetyl(acetoin) reductase from Aerobacter aerogenes—Structural properties. Eur. J. Biochem. 20, 200–205 (1971)
Juni, E.: Mechanisms of formation of acetoin by bacteria. J. Biol. Chem. 195, 715–726 (1952)
Juni, E., Heym, G. A.: Cyclic pathway for the bacterial dissimilation of 2,3-butanediol, acetylmethylcarbinol, and diacetyl. I. General aspects of the 2,3-butanediol cycle. J. Bacteriol. 71, 425–432 (1956)
Larsen, S. H., Størmer, F. C.: Diacetyl(acetoin) reductase from Aerobacter aerogenes; kinetic mechanism and regulation by acetate of the reversible reduction of acetoin to 2,3-butanediol. Eur. J. Biochem. 34, 100–106 (1973)
Løken, J. P., Størmer, F. C.: Acetolactate decarboxylase from Aerobacter aerogenes. Purification and properties. Eur. J. Biochem. 14, 133–137 (1970)
Masuda, H., Muraki, H.: Enzymatic determination of acetoin in wines. J. Sci. Food Agric. 26, 1027–1036 (1975)
Maurer, H. R.: Disk-Elektrophorese. Berlin: de Gruyter 1968
Muraki, H., Masuda, H.: Enzymatic determination of butane-2,3-diol in wines. J. Sci. Food Agric. 27, 345–350 (1976)
Neish, A. C.: Production and properties of 2,3-butanediol. IV. Purity of the levorotatory 2,3-butanediol produced by Aerobacillus polymyxa. Can. J. Res. Sect. B23, 10–16 (1945)
Stanier, R. Y., Fratkin, S. B.: Studies on bacterial oxidation of the 2,3-butanediol and related compounds. Can. J. Res. Sect. B22, 140–153 (1944)
Størmer, F. C.: Isolation of crystalline pH 6 acetolactate-forming enzyme from Aerobacter aerogenes. J. Biol. Chem. 242, 1756–1759 (1967)
Taylor, M. B., Juni, E.: Stereoisomeric specifities of 2,3-butanediol-dehydrogenases. Biochim. Biophys. Acta 39, 448–457 (1960)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Höhn-Bentz, H., Radler, F. Bacterial 2,3-butanediol dehydrogenases. Arch. Microbiol. 116, 197–203 (1978). https://doi.org/10.1007/BF00406037
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00406037