Abstract
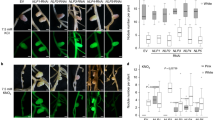
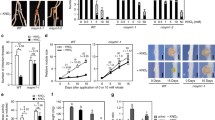
When high dosages of wild-type Rhizobium meliloti RCR2011 were inoculated at two different times, 24 h apart, onto either the primary roots of alfalfa (Medicago sativa L.) seedlings or onto lateral roots on opposite sides of a split-root system, the number of nodules generated by the second inoculum was much smaller than the number generated by the first inoculum. These results provide evidence that alfalfa has an active, systemic mechanism for feedback control of nodulation. Non-nodulating mutants and delayed, weakly nodulating mutants did not elicit a discernable suppression of nodulation by subsequently inoculated wild-type cells. An appreciable number of Rhizobium infections thus seem required to elicit the suppressive response. Mutants in nodulation regions IIb and IIa nodulated extensively in the initially susceptible region of the root, but nodule initiation by these mutants was 100–1000 times less efficient, respectively, than the parent. Nodules formed by these mutants emerged 1 d later than normal. The IIb mutants elicited a relatively strong suppression of nodulation in younger parts of the root, but region-IIa mutants elicited only a weak response. These results indicate that elicitation of the regulatory response need not be proportional to nodule formation and imply that genes in region IIa play an important role in elicitation. At high dosages, the region-II mutants induced the development of thick, short roots in a considerably higher percentage of plants than the wild-type bacteria. Nodules generated by wild-type isolates and region-II mutants did not emerge in strict acropetal sequence, probably because some infections developed more slowly than others. Prior exposure of the root to non-nodulating mutants resulted in nodulation by the parent in regions of the root otherwise too mature to be susceptible, indicating that exposure to these mutants may affect the sequence of root development.
Similar content being viewed by others
Abbreviations
- RT:
-
root tip
- EH:
-
smallest emergent root hair
- Tsr:
-
thick, short roots
References
Allen, O.N., Allen, E.K. (1981) The Leguminosae, a source book of characteristics, uses and nodulation. University of Wisconsin Press, Madison, USA
Bhuvaneswari, T.V., Bhagwat, A.A., Bauer, W.D. (1981) Transient susceptibility of root cells in four common legumes to nodulation by rhizobia. Plant Physiol. 68, 1114–1149
Bhuvaneswari, T.V., Lesniak, A.P., Bauer, W.D. (1988) Efficiency of nodule initiation in cowpea and soybean. Plant Physiol. 86, 1210–1215
Bhuvaneswari, T.V., Mills, K.K., Crist, D.K., Evans, W.R., Bauer, W.D. (1983) Effects of culture age on symbiotic infectivity of Rhizobium japonicum. J. Bacteriol. 153, 443–451
Bhuvaneswari, T.V., Turgeon, G., Bauer, W.D. (1980) Early stages in the infection of soybean (Glycine max L. Merr.) by Rhizobium japonicum. I. Localization of infectible root cells. Plant Physiol. 66, 1027–1031
Caetano-Anollés, G., Bauer, W.D. (1988) Enhanced nodule initiation on alfalfa by wild-type Rhizobium meliloti co-inoculated with nod gene mutants and other bacteria. Planta 174, 385–395
Caetano-Anollés, G., Favelukes G. (1986) Host-symbiont specificity expressed during early adsorption of Rhizobium meliloti to the root surface of alfalfa. Appl. Environ. Microbiol. 52, 377–382
Caetano-Anollés, G., Wall, L.G., DeMicheli, A.T., Macchi, E.M., Bauer, W.D., Favelukes, G. (1988) Role of motility and chemotaxis in efficiency of nodulation by Rhizobium meliloti. Plant Physiol 86, 1228–1235
Calvert, H.E., Pence, M.K., Pierce, M., Malik, N.S.A., Bauer, W.D. (1984) Anatomical analysis of the development and distribution of Rhizobium infections in soybean roots. Can. J. Bot. 62, 2375–2384
Carroll, B.J., McNeil, D.L., Gresshoff, P.M. (1985) Isolation and properties of soybean (Glycine max. (L) Merr.) mutants that nodulate in the presence of high nitrate concentrations. Proc. Natl. Acad. Sci. USA 82, 4162–4166
Debellé, F., Rosenberg, C., Vasse, J., Maillet, F., Martinez, E., Dénarié, J., Truchet, G. (1986) Assignment of symbiotic development phenotypes to common and specific nodulation (nod) genetic loci of Rhizobium meliloti. J. Bacteriol. 168, 1075–1086
Delves, A.C., Mathews, A., Day, D.A., Carter, A.S., Carroll, B.J., Gresshoff, P.M. (1986) Regulation of the soybean-Rhizobium symbiosis by shoot and root factors. Plant Physiol. 82, 588–590
Dudley, M.E., Jacobs, T.W., Long, S.R. (1987) Microscopic studies of cell divisions induced in alfalfa roots by Rhizobium meliloti. Planta 171, 289–301
Heron, D.S., Pueppke, S.G. (1987) Regulation of nodulation in the soybean-Rhizobium symbiosis. Strain and cultivar variability. Plant Physiol. 84, 1391–1396
Hirsch, A.M., Long, S.R., Bang, M., Haskins, N., Ausubel, F.M. (1982) Structural studies of alfalfa roots infected with nodulation mutants of Rhizobium meliloti. J. Bacteriol. 151, 411–419
Hoagland, D.R., Arnon, D.I. (1950) The water-culture method for growing plants without soil. Cal. Agric. Exp. Stat., Circ. No. 347 (rev. edn.)
Jensen, H.L. (1942) Nitrogen fixation in leguminous plants. I. General characters of root nodule bacteria isolated from species of Medicago and Trifolium in Australia. Proc. Linn. Soc. N.S.W. 66, 98–108
Kosslak, R.M., Bohlool, B.B. (1984) Suppression of nodule development of one side of a split-root system of soybeans caused by prior inoculation of the other side. Plant Physiol. 75, 125–130
Meade, H.M., Long, S.R., Ruvkun, G.B., Brown, S.E., Ausubel, F.M. (1982) Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon mutagenesis. J. Bacteriol. 149, 114–122
Peterson, M.A., Barnes, D.K. (1981) Inheritance of ineffective nodulation and non-nodulation traits in alfalfa. Crop Sci. 21, 611–616
Pierce, M., Bauer, W.D. (1983) A rapid regulatory response governing nodulation in soybean. Plant Physiol. 73, 286–290
Rosenberg, C., Boistard, P., Dénarié, J., Casse-Delbart, F. (1981) Genes controlling early and late functions in symbiosis are located on a megaplasmid in Rhizobium meliloti. Mol. Gen. Genet. 184, 326–333
Sargent, L., Huang, S.Z., Rolfe, B.G., Djordjevic, M.A. (1987) Split-root assays using Trifolium subterraneum show that Rhizobium infection induces a systemic response that can inhibit nodulation of another invasive Rhizobium strain. Appl. Environ. Microbiol. 53, 1611–1619
Takats, S.T. (1986) Suppression of nodulation in soybeans by superoptimal inoculation with Bradyrhizobium japonicum. Physiol. Plant. 66, 669–673
Truchet, G., Debellé, F., Vasse, J., Terzaghi, B., Garnerone, A.M., Rosenberg, C., Batut, J., Maillet, F., Dénarié, J. (1985) Identification of a Rhizobium meliloti pSym 2011 region controlling the host specificity of root hair curling and nodulation. J. Bacteriol. 164, 1200–1210
Truchet, G., Rosenberg, C., Vasse, J., Julliot, J.S., Camut, S., Dénarié, J. (1984) Transfer of Rhizobium meliloti pSym genes into Agrobacterium tumefaciens: host-specific nodulation by atypical infection. J. Bacteriol. 157, 134–142
van Brussel, A.A.N., Tak, T., Wetselaar, A., Pees, E., Wijffelman, C.A. (1982) Small leguminosae as test plants for nodulation of Rhizobium leguminosarum and other Rhizobia and Agrobacteria harbouring a leguminosarum Sym-plasmid. Plant Sci. Lett. 27, 317–325
van Brussel, A.A.N., Zaat, S.A.J., Canter Cremers, H.C.J., Wijffelman, C.A., Pees, E., Tak, T., Lugtenberg, B.J.J. (1986) Role of plant root exudate and Sym plasmid-localized nodulation genes in the synthesis by Rhizobium leguminosarum of Tsr factor, which causes thick and short roots on common vetch. J. Bacteriol. 165, 517–522
Author information
Authors and Affiliations
Additional information
This is contribution No. 79-88 of the Ohio Agricultural Research and Development Center
Rights and permissions
About this article
Cite this article
Caetano-Anollés, G., Bauer, W.D. Feedback regulation of nodule formation in alfalfa. Planta 175, 546–557 (1988). https://doi.org/10.1007/BF00393078
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00393078